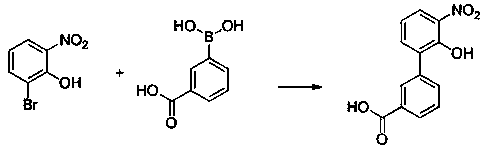
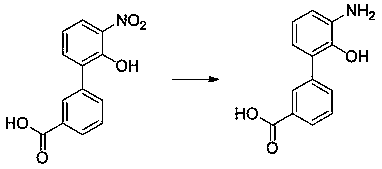
Preparation method of Eltrombopag key intermediate 3'-amino-2'-hydroxybiphenyl-3-carboxylic acid
A technology of hydroxybiphenyl and intermediates, applied in the field of organic and pharmaceutical synthesis, can solve the problems of inapplicability to mass production, high processing costs, and cumbersome steps, and achieve the effects of simple post-processing, low price, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The following are specific examples of the present invention to further describe the technical solutions of the present invention, but the present invention is not limited to these examples.
[0025] The synthetic route of the present invention is as follows:
[0026]
[0027] Preparation of 2-bromo-6-nitrophenol
[0028] Take 5ml (0.078mol) of concentrated sulfuric acid and slowly add it into 16ml of water along the wall of the cup, add 6.6g (0.078mol) of sodium nitrate into the diluted sulfuric acid, stir well until it is completely dissolved. Under the condition of ice bath, slowly add 7.46g (0.043mol) of o-bromophenol dropwise to the above solution, and remove the ice bath after dropping. Reaction for 2 hours, followed by TLC until the reaction was complete, filtered, the filter cake was washed with water (5ml×4), dissolved in ethyl acetate, dried over anhydrous magnesium sulfate, filtered, the filtrate was concentrated under reduced pressure, and the resulting ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com