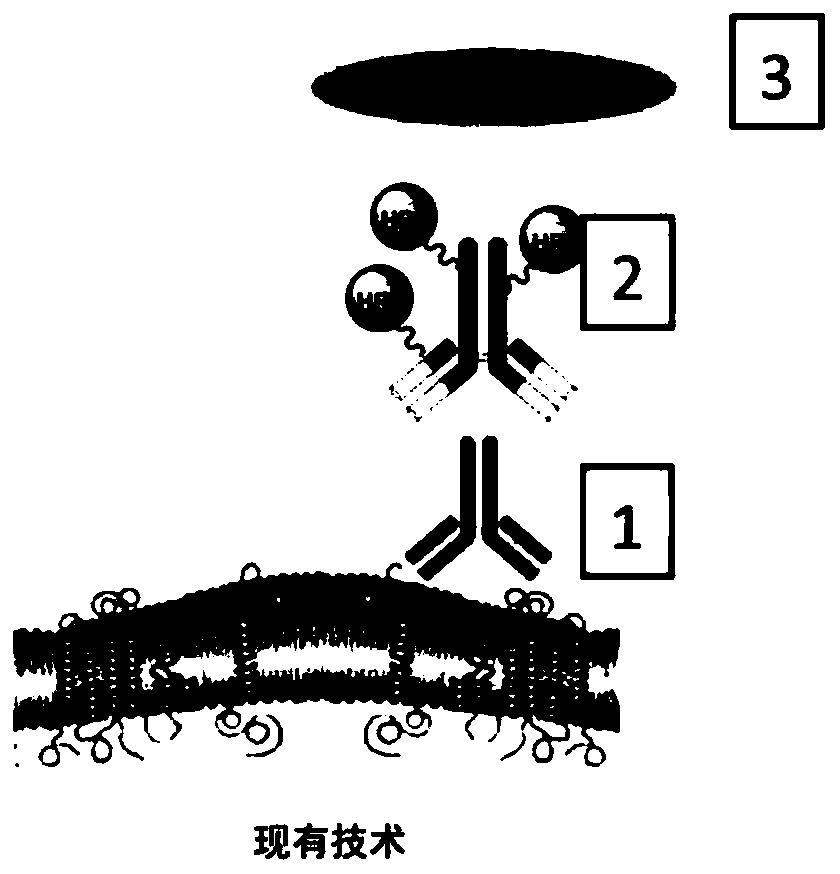
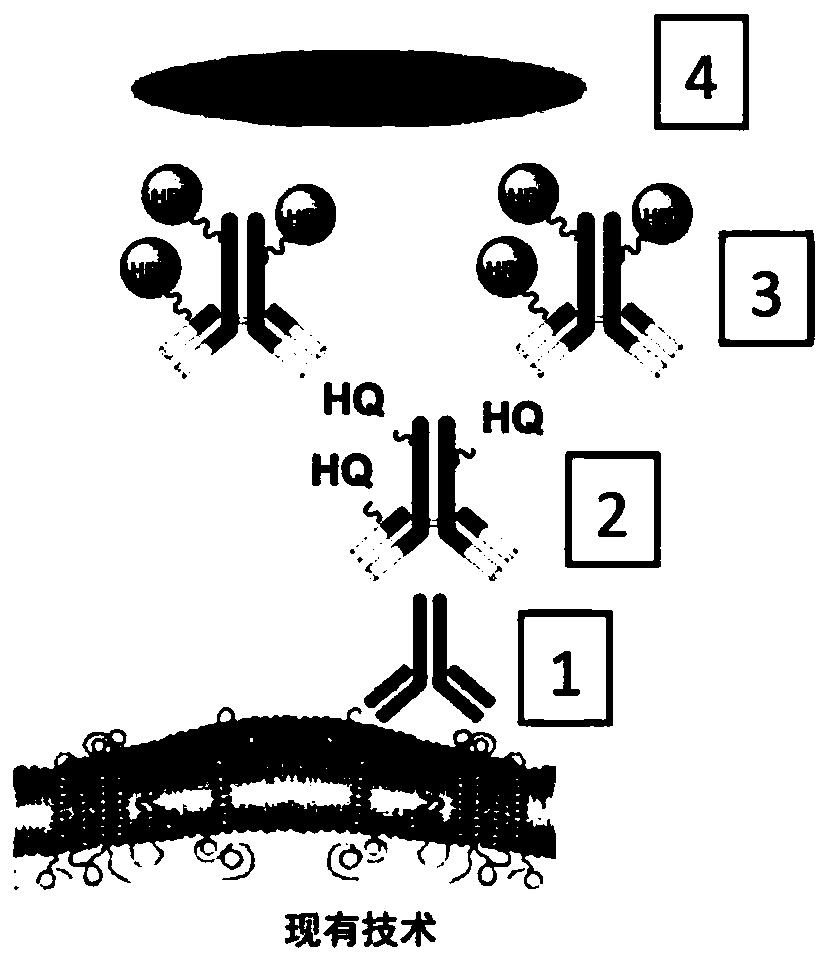
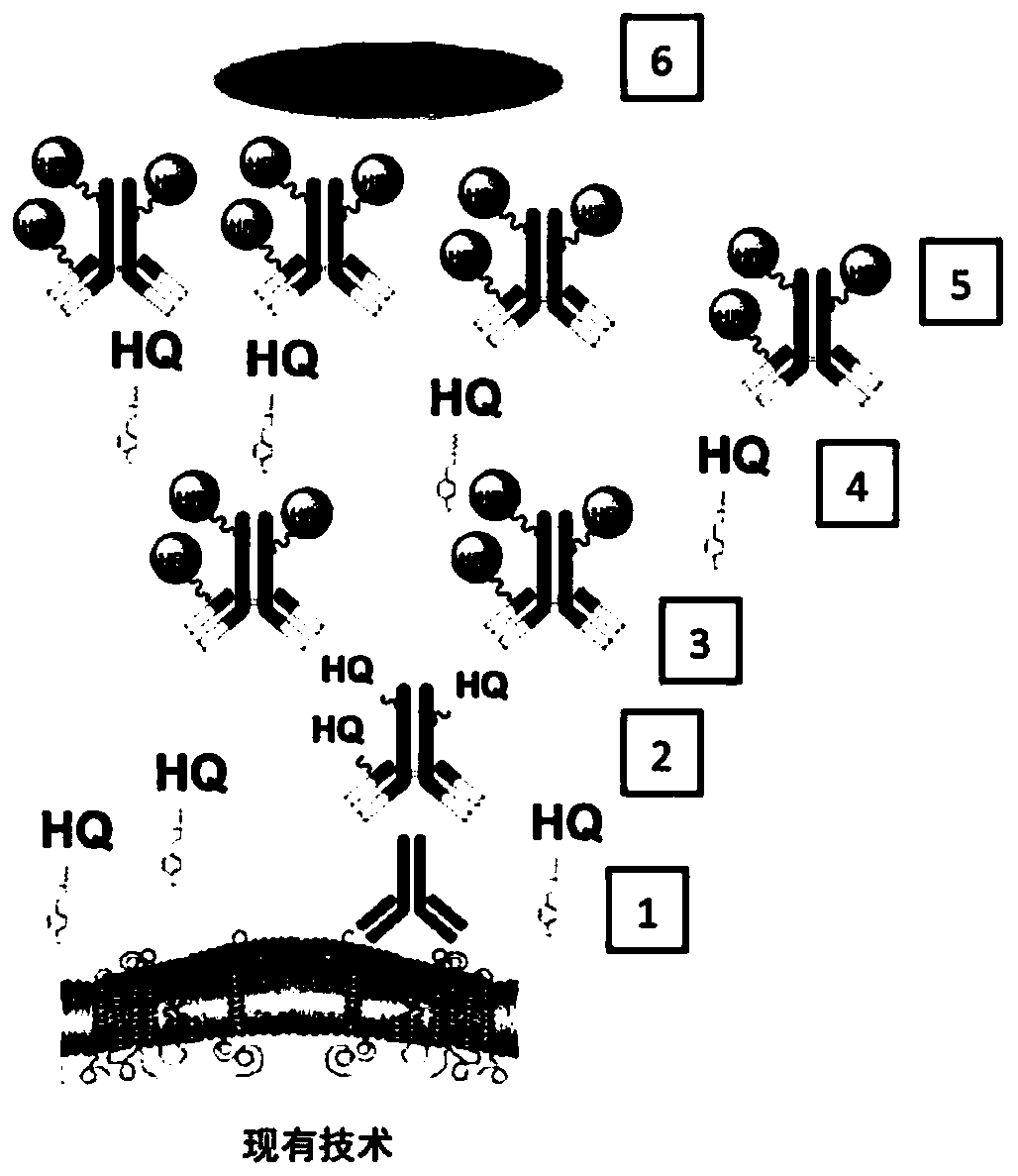
Methods and systems for quantitative immunohistochemistry
A tissue sample, peroxidase technology, used in biochemical equipment and methods, measurement devices, scientific instruments, etc., can solve the problems of reflecting the presence of signals, inability to detect or quantify individual protein molecules, and deletions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0155] Example 1 describes a series of experiments using the method of the present invention. The present invention is not limited to the methods, systems, and compositions described herein.
[0156] Experiment 1: A high-sensitivity detection system (a method of the present invention) was assembled, including 4B5 anti-Her2 rabbit monoclonal antibody with V5 epitope tag + mouse anti-V5-HRP conjugate + tyramide-DIG + Mouse anti-DIG-HRP conjugate + silver chromophore. Determination of the concentration of anti-V5-HRP conjugate capable of specific (little or no background) and sensitive detection of anti-Her2 antibody bound to Her2 expressing cells in each tissue sample was achieved using titration experiments. Tonsil and Her2 triple xenograft slides were stained using V5 epitope-tagged 4B5 anti-Her2 rabbit monoclonal antibody or a negative control (antibody diluent) and a range of anti-V5-HRP conjugate concentrations. Similar experiments were performed to demonstrate specificit...
Embodiment 2
[0162] See Figure 9 , Tonsil tissue was stained for Her2 using a multiplex staining assay. Anti-Her2 monoclonal antibody 4B5 tagged with V5 epitope was detected using mouse anti-V5-HRP+tyramide-DIG+mouse anti-DIG-HRP+tyramide-TAMRA chromophore (pink). Anti-Her2 monoclonal antibody 4B5 tagged with E2 epitope was detected using mouse anti-E2-HRP+tyramine-NP+mouse anti-NP-HRP+silver chromophore (black). Note that the V5 and E2 tagged 4B5 anti-Her2 monoclonal antibodies compete for the same amino acid sequence in the target Her2 protein and were co-incubated together before performing the assay. Single pink dots signal, most likely derived from a single single V5-labeled 4B5 mAb bound to the target; each antibody likely binds only a single Her2 protein. Figure 9 Consistent with detection of individual antibodies binding to individual epitopes (eg, single protein molecule detection). Figure 10 Detection of Her2 protein in tonsil tissue is shown, demonstrating the specificity ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com