Method for producing dialkylaminosilane
A technology of alkylaminosilane and its manufacturing method, which is applied in chemical instruments and methods, compounds of group 4/14 elements of the periodic table, organic chemistry, etc., can solve problems such as volume efficiency reduction, and prevent runaway reactions and reduce halogen content Less, good yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068]
[0069]
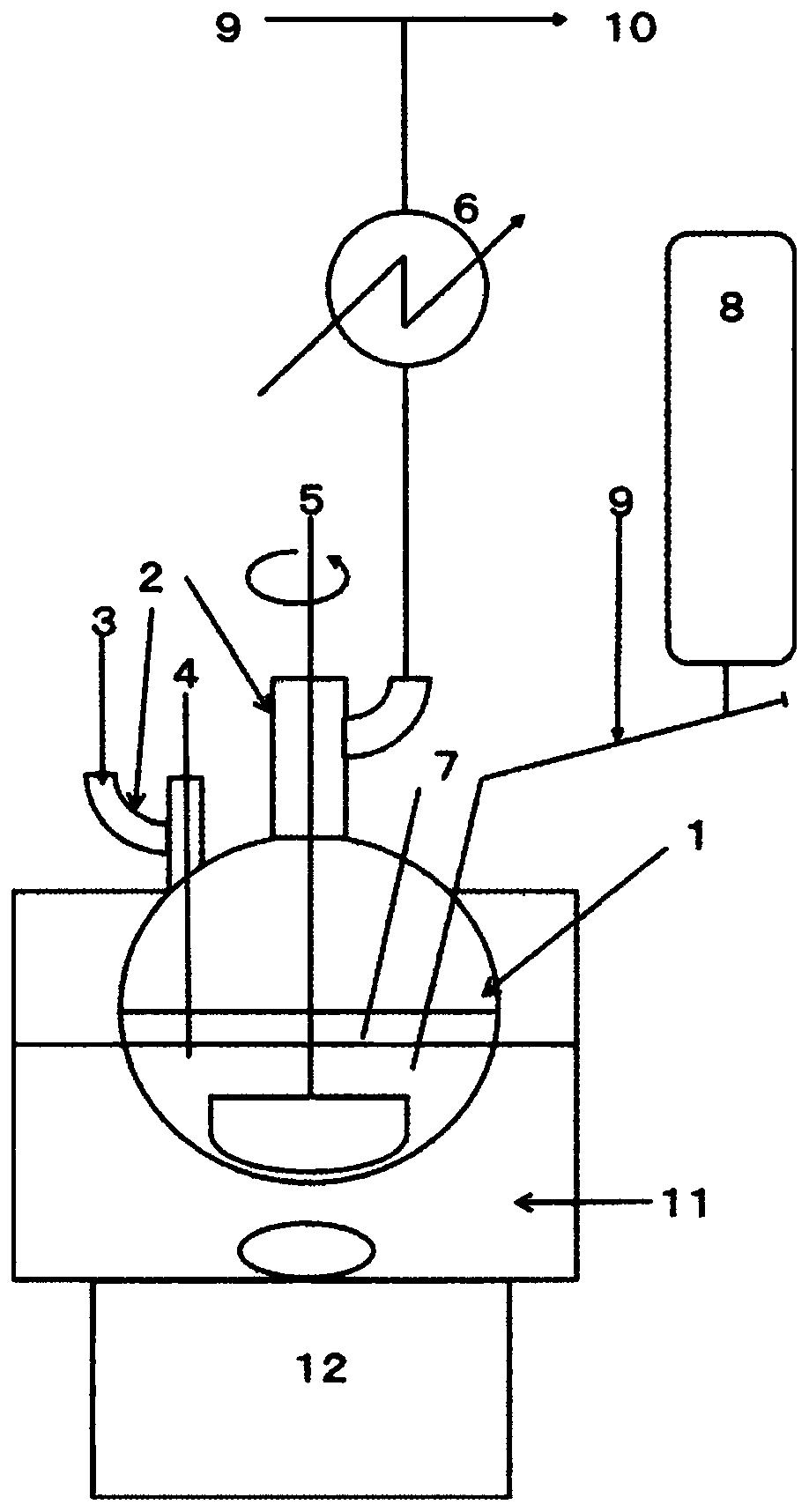
[0070] 7.66 g (0.32 mol) of magnesium and 90 g of n-heptane were placed in a 300 mL four-necked flask. While stirring, the oil bath was heated to 115° C., kept in a reflux state for 1 hour, and the solvent and moisture in the apparatus were reacted with magnesium to perform dehydration. After that, the oil bath was left to cool with ice water. 40.8 g (30 mL, 0.30 mol) of trichlorosilane (TCS) was charged in a 50 mL supply tank, and when the internal temperature dropped to 5° C., 3 mL of TCS was charged into a 300 mL four-necked flask. Dimethylamine (DMA) was supplied from the gas phase portion of the flask at a rate of 16 mL per minute while maintaining 10° C. or lower for 1 hour.
[0071]
[0072] The cooling with ice water was stopped, and the 300 mL 4-necked flask was immersed in the oil bath again, the oil temperature was set to 90° C., and the temperature of the reaction solution was heated to 80° C. or higher. During the heating, the reaction be...
Embodiment 2
[0078]
[0079]
[0080] 7.64 g (0.31 mol) of magnesium, 60 g of n-heptane, and 30 g of 1,2-dimethoxyethane (DME) were placed in a 300 mL four-necked flask. While stirring, the oil bath was heated to 115° C. and kept under reflux for 1 hour to dehydrate the solvent and moisture in the apparatus by reacting with magnesium, and then cooled with ice water. 45.0 g (32 mL, 0.33 mol) of TCS was charged into a 50 mL supply tank, and when the internal temperature dropped to 5° C., 3 mL of TCS was charged into a 300 mL four-necked flask. DMA was supplied from the gas phase portion of the flask at a rate of 37 mL per minute while maintaining 10° C. or lower for 1 hour.
[0081]
[0082]The oil bath was set at 90°C, and the temperature of the reaction solution was heated to above 80°C. During the heating, the reaction between the hydrochloride salt of DMA and magnesium occurred, but a significant rapid rise in temperature was not observed. When the internal temperature exceeded ...
Embodiment 3
[0088]
[0089]
[0090] 5.10 g (0.21 mol) of magnesium, 60 g of n-heptane, and 15 g of THF were placed in a 300 mL four-necked flask. While stirring, the oil bath was heated to 115° C. and kept under reflux for 1 hour to dehydrate the solvent and moisture in the apparatus by reacting with magnesium, and then cooled with ice water. 28.0 g (20 mL, 0.21 mol) of TCS was charged into a 50 mL supply tank, and when the internal temperature dropped to 5° C., 3 mL of TCS was charged into a 300 mL four-necked flask. While maintaining 10° C. or lower, DMA was supplied from the gas phase portion of the flask at a rate of 16 mL per minute for 1 hour.
[0091]
[0092] The oil bath was set at 90°C, and the temperature of the reaction solution was heated to above 80°C. During the heating, the reaction between the hydrochloride salt of DMA and magnesium occurred, but a significant rapid rise in temperature was not observed. When the internal temperature exceeded 80° C., TCS was adde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com