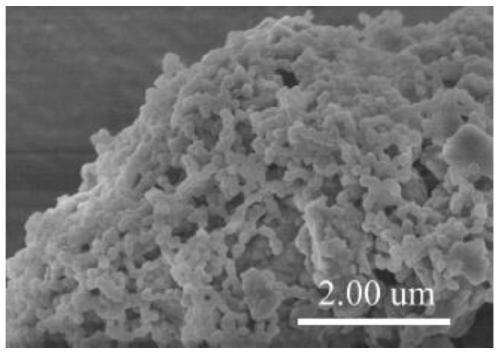
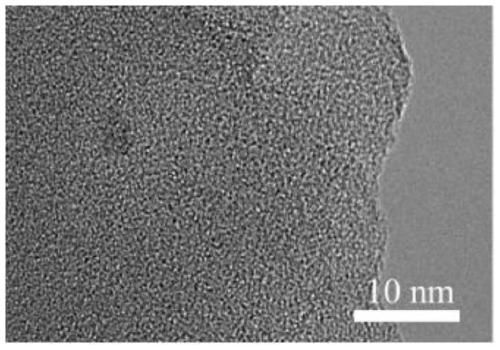
Preparation method of conjugated three-dimensional porphyrin-based covalent organic framework material
A covalent organic framework and three-dimensional porphyrin-based technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, etc., can solve porosity reduction and three-dimensional structure interpenetration , less research work and other issues, to achieve the effect of improving the performance of biomimetic catalysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation method of the conjugated three-dimensional porphyrin-based covalent organic framework material in this example is as follows:
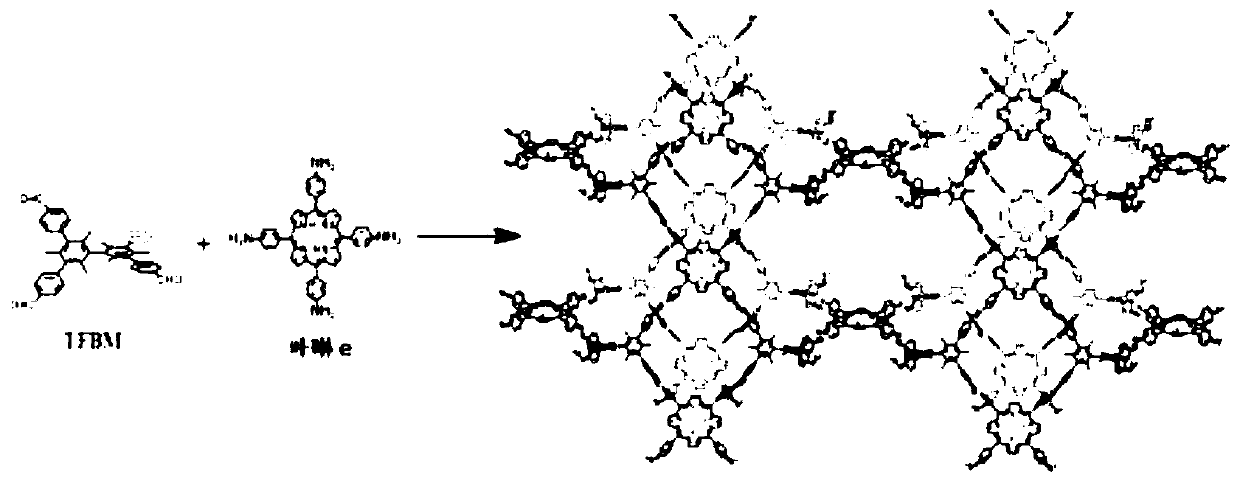
[0028] (1) Mix 0.3mmol of 3,3',5,5'-tetrakis(4-formylphenyl)hexamethylbiphenyl TFBM (from Shanghai Kaishu Chemical Technology Co., Ltd.) with 0.3mmol of porphyrin e (synthetic diagram see figure 1 ) was added to a mixed solvent of 1mL o-dichlorobenzene and 1mL n-butanol, and dispersed evenly by ultrasonication.
[0029] (2) Add 0.2 mL of 6 mol / L acetic acid as a catalyst into the uniformly dispersed solution, put it into the reaction kettle, and put it into the reaction kettle after ultrasonically dispersing evenly.
[0030] (3) The reaction kettle was kept at a constant temperature of 110° C. for 5 days, cooled to room temperature after the reaction, and the solid was collected by filtration.
[0031] (4) The collected solid was centrifuged and washed 10 mL×5 times with DMF and THF successively, and the solid was collected ag...
Embodiment 2
[0039] The preparation method of the conjugated three-dimensional porphyrin-based covalent organic framework material in this example is as follows:
[0040] (1) Mix 0.3mmol of 3,3’,5,5’-tetrakis(4-formylphenyl)hexamethylbiphenyl TFBM with 0.2mmol of porphyrin e (see figure 1 ) was added to a mixed solvent of 2mL o-dichlorobenzene and 1mL n-butanol, and dispersed evenly by ultrasonic.
[0041] (2) Add 0.4 mL of 5 mol / L acetic acid as a catalyst into the uniformly dispersed solution, put it into the reaction kettle, and put it into the reaction kettle after ultrasonically dispersing evenly.
[0042] (3) The reaction kettle was kept at a constant temperature of 120° C. for 6 days, cooled to room temperature after the reaction, and the solid was collected by filtration.
[0043] (4) The collected solid was centrifuged and washed 15 mL×6 times with DMF and THF successively, and the solid was collected again.
[0044](5) After Soxhlet extraction of solid THF for 24 hours, vacuum ...
Embodiment 3
[0047] The preparation method of the conjugated three-dimensional porphyrin-based covalent organic framework material in this example is as follows:
[0048] (1) Mix 0.3mmol of 3,3’,5,5’-tetrakis(4-formylphenyl)hexamethylbiphenyl TFBM with 0.1mmol of porphyrin e (see figure 1 ) was added to a mixed solvent of 3mL o-dichlorobenzene and 1mL n-butanol, and dispersed evenly by ultrasonication.
[0049] (2) Add 0.5 mL of 4 mol / L acetic acid as a catalyst into the uniformly dispersed solution, put it into the reaction kettle, and put it into the reaction kettle after ultrasonically dispersing evenly.
[0050] (3) The reaction kettle was kept at a constant temperature of 115° C. for 6 days, cooled to room temperature after the reaction, and the solid was collected by filtration.
[0051] (4) The collected solid was centrifuged and washed 20 mL×7 times with DMF and THF successively, and the solid was collected again.
[0052] (5) After Soxhlet extraction of the solid THF for 24 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com