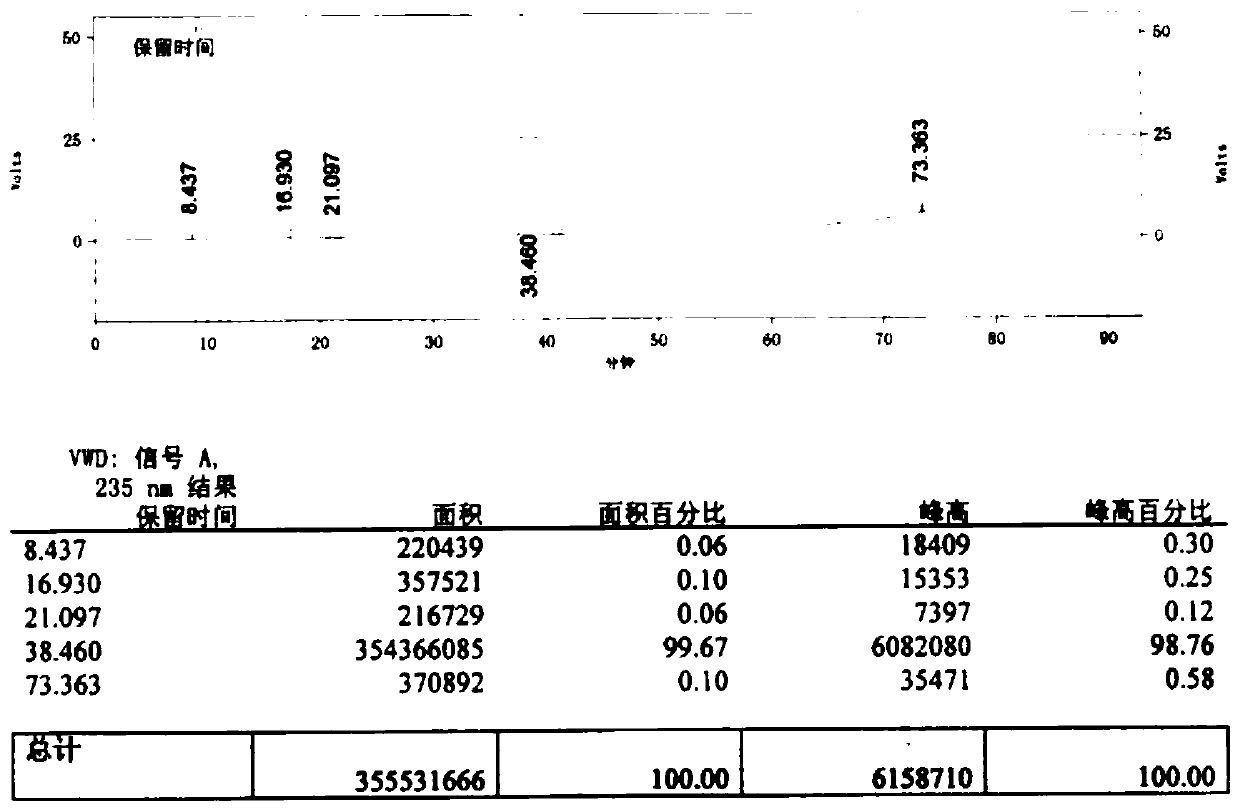
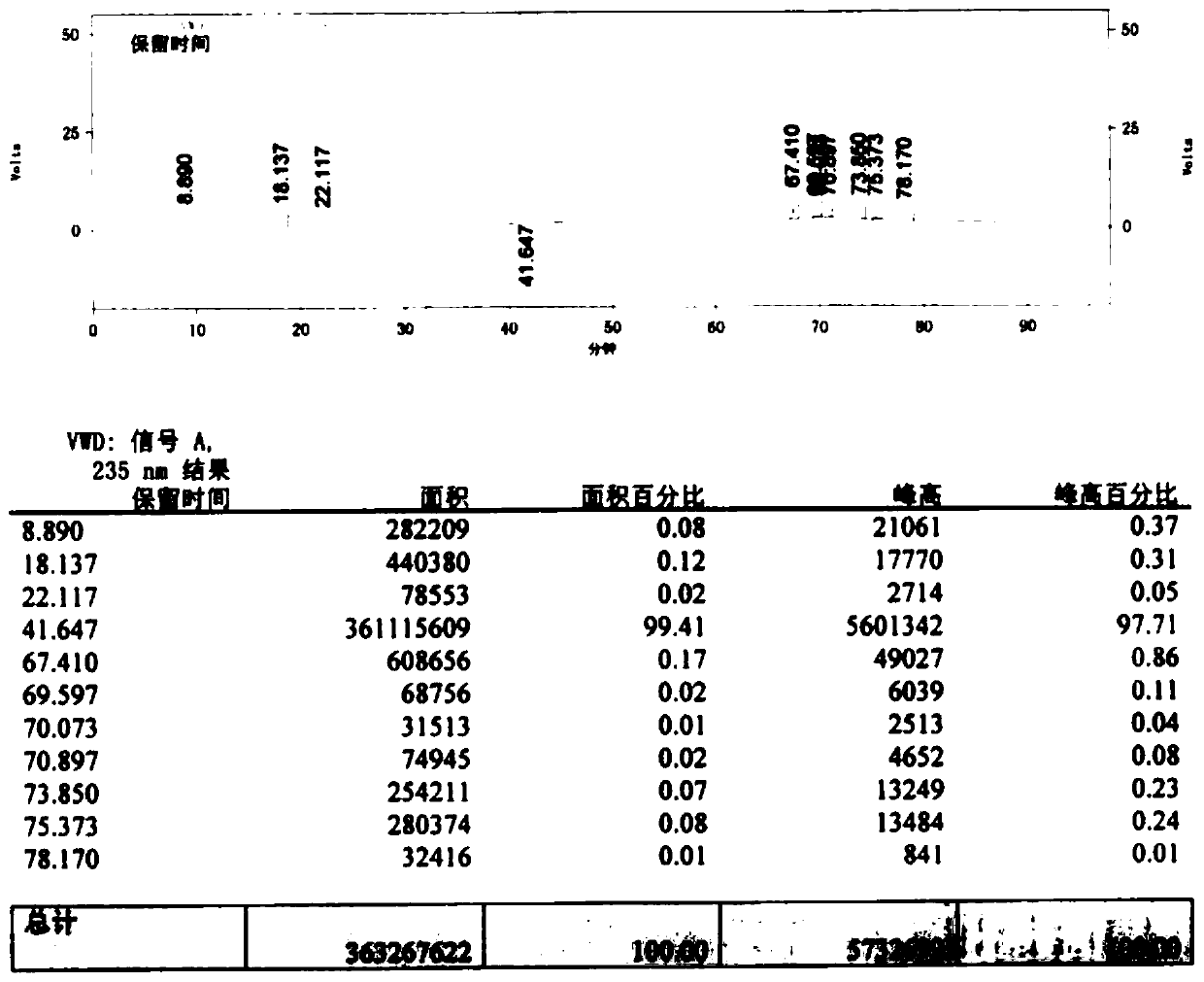
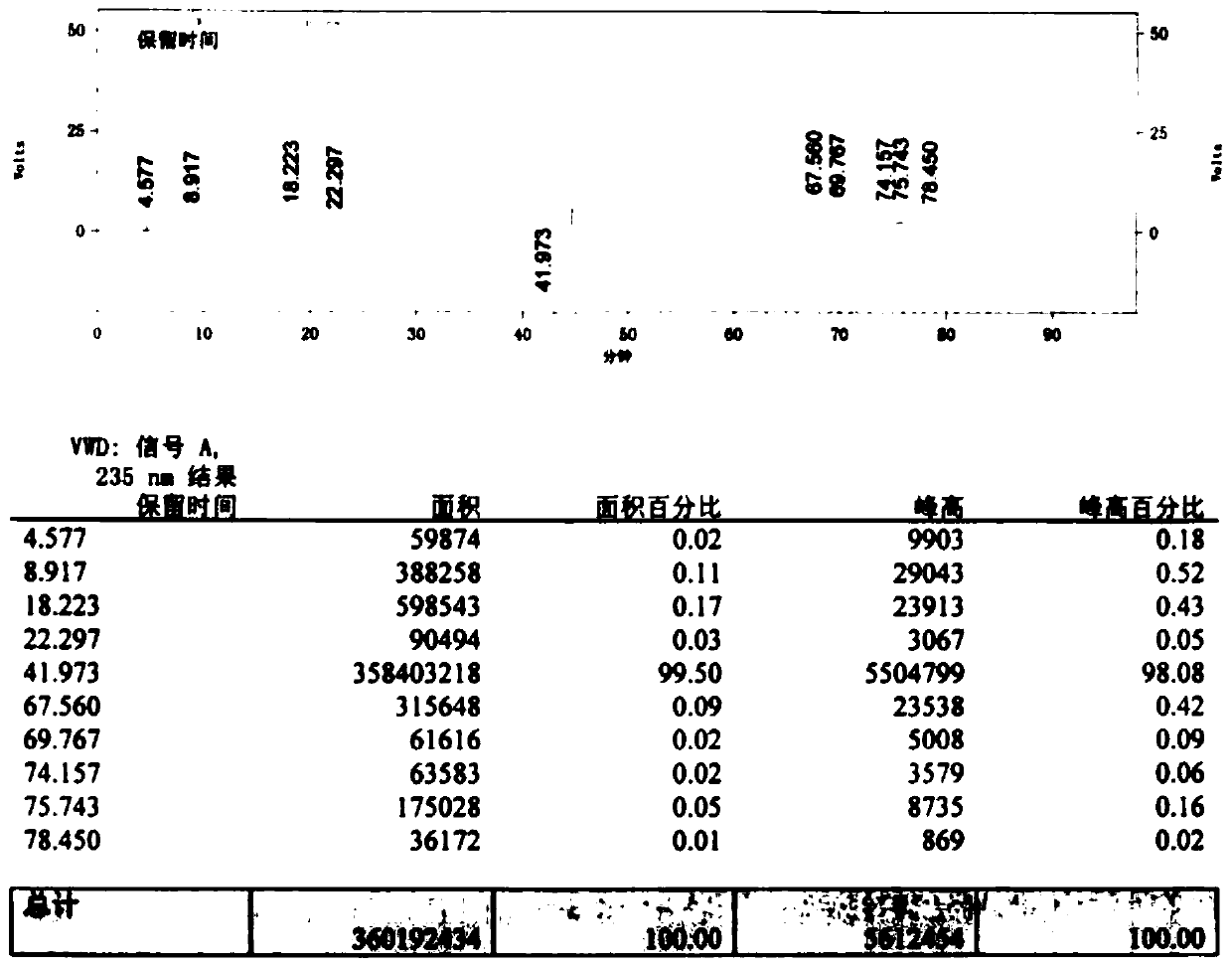
Preparation method for improving product quality of cefotaxime sodium
A technology for cefotaxime sodium and cefotaxime acid, which is applied in the field of organic synthesis, can solve the problems of unfavorable production, research and utilization, high product color grade and high product impurities, and achieves the improvement of product market competitiveness, high content, high color good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Add 400ml of chloroform to a dry and clean four-neck bottle, add 20g of 7-aminocephalosporanic acid, 5g of methanol, and 5g of pure water at a temperature controlled below 10°C, add DIPEA dropwise at a temperature controlled to pH7.0-9.0, and then statically separate layers , to separate the upper layer of organic impurities. Control the temperature below 20°C and add 26g of AE-active ester to condense for 2-5hr.
[0050] Then add 30ml of pure water for extraction, add 60ml of ethyl acetate to the aqueous phase and stir for 5-10min, then static and separate layers. Add 8.3 g of sodium isooctanoate / 120 ml of absolute ethanol to the aqueous phase, decolorize with activated carbon for 20 minutes and filter. The temperature of the filtrate was controlled below 30°C, 160ml of acetone was added, and the crystal was grown for 60min. After adding 500ml of acetone dropwise, wait until the crystals are completely precipitated. Filter, drain and then wash with acetone, drain and ...
Embodiment 2
[0053] Add 300ml of dichloromethane to a dry and clean four-neck bottle, add 20g of 7-aminocephalosporanic acid, 10g of ethanol, and 8g of pure water under temperature control below 10°C, add TEA dropwise under temperature control to pH7.0-9.0, and then statically separate layers , to separate the upper layer of organic impurities. Control the temperature below 20°C and add 27g of AE-active ester to condense for 2-5hr.
[0054] Then add 50ml of pure water for extraction, add 100ml of 2-methyltetrahydrofuran into the water phase, stir for 5-10min, and then separate the layers. Add 6.5 g of sodium acetate to the aqueous phase and stir for 10 min, decolorize with activated carbon for 15 min and filter. The temperature of the filtrate was controlled below 30°C, 280ml of acetone was added, and the crystal was grown for 60min. Add 850ml of acetone in the later stage until the crystals are completely precipitated. Filter, drain and then wash with acetone, drain and dry to obtain 2...
Embodiment 3
[0057] Add 200ml of carbon tetrachloride to a dry and clean four-necked bottle, add 20g of 7-aminocephalosporanic acid, 2.5g of ethanol, and 2.5g of pure water under temperature control below 10°C, and add ethylenediamine dropwise under temperature control to pH7.0-9.0 , and then static layered, separate the upper layer of organic impurities. Control the temperature below 20°C and add 28g of AE-active ester to condense for 2-5hr.
[0058] Then add 60ml of pure water for extraction, add 120ml of ethyl acetate to the aqueous phase and stir for 5-10min, then static and separate layers. Add 8.3 g of sodium isooctanoate / 120 ml of absolute ethanol to the water phase, decolorize with activated carbon for 15 minutes and filter. The temperature of the filtrate was controlled below 30°C, 325ml of acetone was added, and the crystal was grown for 60min. Add 900ml of acetone in the later stage until the crystals are completely precipitated. Filter, drain and then wash with acetone, drai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com