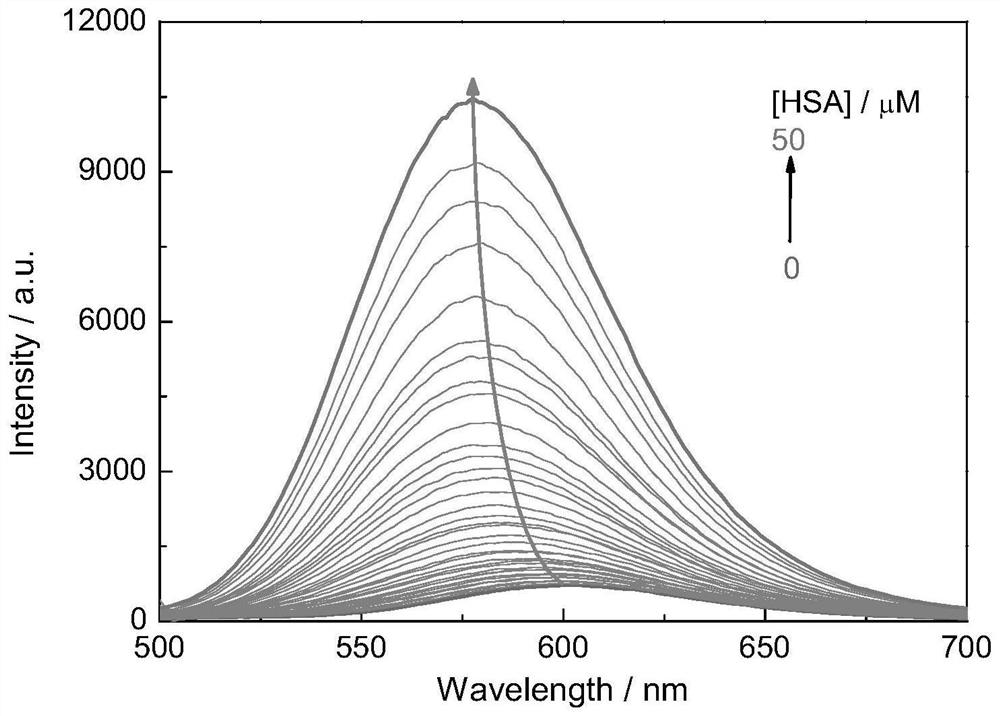
Fluorescent probe for detecting human serum albumin and its synthesis method and application
A technology of serum albumin and fluorescent probes, applied in the synthesis of the above-mentioned fluorescent probes, the application in the detection of human serum albumin, a method and strategy for detecting human serum albumin, which can solve the problems of low sensitivity and achieve The effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] The invention also discloses a method for synthesizing a fluorescent probe for detecting human serum albumin, which specifically includes the following steps:
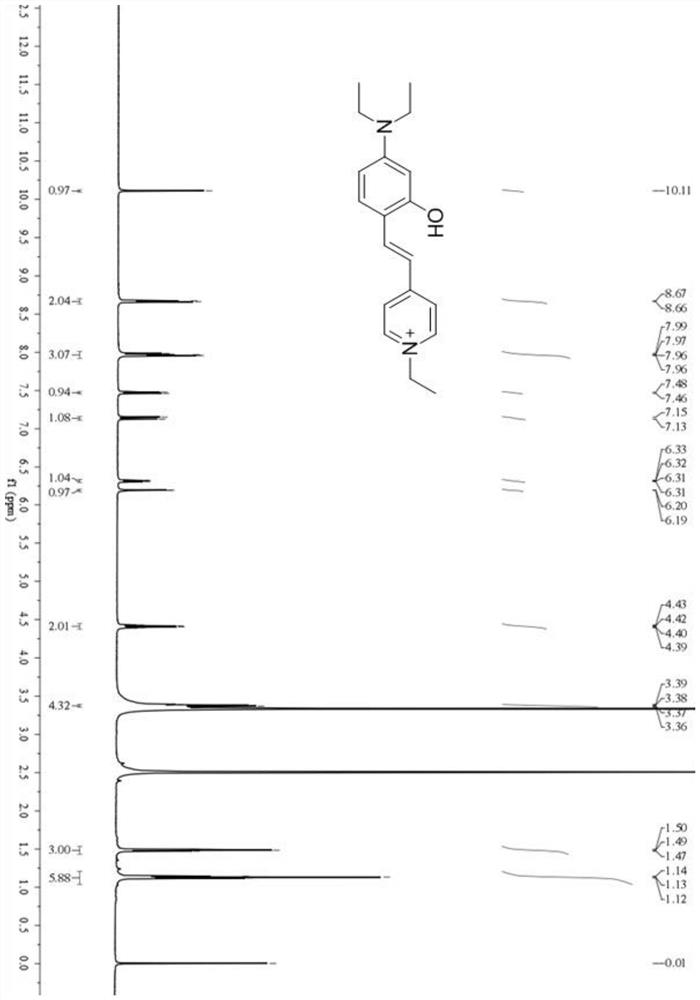
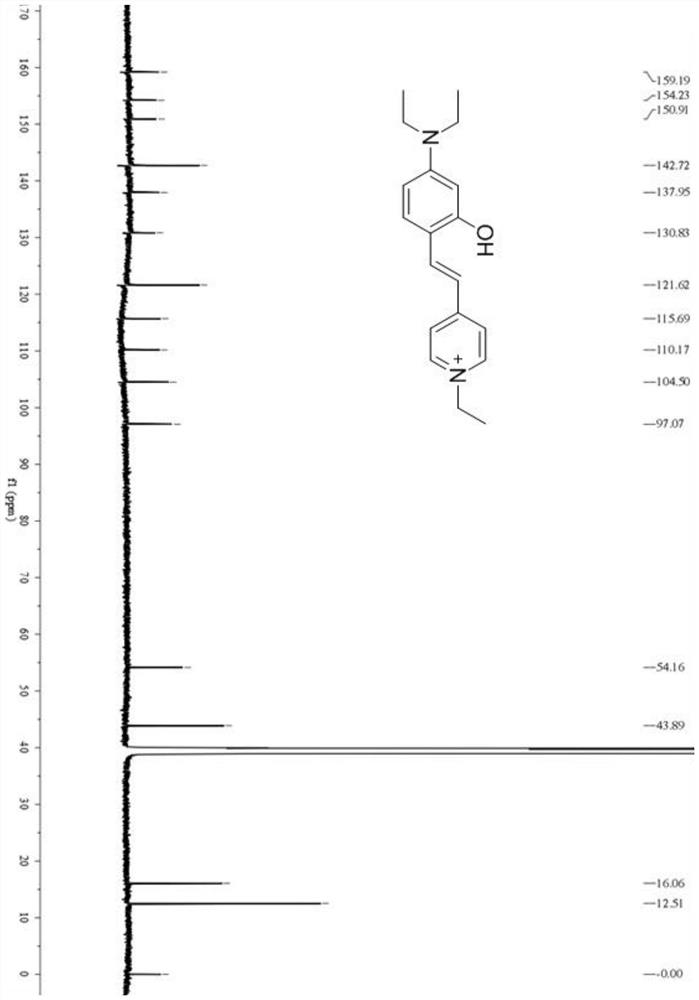
[0055] With ethanol as the reaction medium, 2-hydroxy-4-diethylaminobenzaldehyde and 1-ethyl-4-methylpyridinium iodide were refluxed at 60°C to 80°C for 4 to 5 hours, then distilled under reduced pressure to remove Solvent ethanol to obtain the reaction crude product; add methylene chloride solvent to the reaction crude product, after the chromatographic column purification, the solvent is removed by vacuum distillation to obtain the target pure product, which is the fluorescent probe DMHP synthesized by the present invention.
[0056] In order to further optimize the above technical scheme, the molar ratio of 2-hydroxyl-4-diethylaminobenzaldehyde to 1-ethyl-4-methylpyridine iodide is 1:(0.8~1.2).
[0057] In order to further optimize the above technical scheme, the composition of the eluent during the purificat...
Embodiment 1
[0060] A method for synthesizing a fluorescent probe for detecting human serum albumin, specifically comprising the steps of:
[0061] With ethanol as the reaction medium, 2-hydroxy-4-diethylaminobenzaldehyde and 1-ethyl-4-methylpyridinium iodide were refluxed at 60°C for 4 hours at a molar ratio of 1:0.8, and then distilled under reduced pressure , remove the solvent ethanol to obtain the reaction crude product; add dichloromethane solvent to the reaction crude product, after chromatographic column purification, the solvent is removed by vacuum distillation to obtain the target pure product, which is the fluorescent probe DMHP synthesized by the present invention.
Embodiment 2
[0063] A method for synthesizing fluorescent probes for detecting human serum albumin:
[0064] The difference between this embodiment and Example 1 is that the 2-hydroxyl-4-diethylaminobenzaldehyde in Example 1 is replaced with 1-ethyl-4-methylpyridine iodide at a molar ratio of 1:0.8 The molar ratio of 2-hydroxy-4-diethylaminobenzaldehyde and 1-ethyl-4-methylpyridinium iodide is 1:1.0, and the remaining reactants and experimental parameters refer to Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com