Cationic lipids for nucleic acid delivery and preparation thereof
A technology of cationic lipids and fatty acid residues, which is applied in the field of cationic lipids for nucleic acid delivery and their preparation, and can solve problems related to nucleic acids and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0261] In additional embodiments, the invention relates to cationic lipids comprising a functional group represented by the structure:
[0262] -W-(T=O) m -X-(CH 2 ) z -Y-
[0263] in
[0264] X and Y are each independently O or N, where both X and Y cannot be O;
[0265] W is a bond, O, NH or S;
[0266] T is C or S;
[0267] m is 0 or 1; and
[0268] z is 0 or 2;
[0269] wherein the functional group is attached to at least one saturated or unsaturated fatty acid residue.
[0270] In some embodiments, the cationic lipid comprises two fatty acid residues linked symmetrically or asymmetrically to the functional groups described above.
[0271] In one aspect of the invention, the cationic lipid is represented by the structure of formula (I'):
[0272]
[0273] in
[0274] Y is O or NH;
[0275] T is C or S;
[0276] W is a bond, O, NH or S;
[0277] R 1 Selected from the group consisting of:
[0278] (a) NR 4 R 5 , where R 4 and R 5 each independently is ...
Embodiment 1
[0432] Example 1: Materials and methods:
[0433] Material
[0434] Lipids: All lipids (cholesterol, DSPC and DSPE PEG-maleimide) used for LNP production were purchased from Avanti Polar lipids (USA).
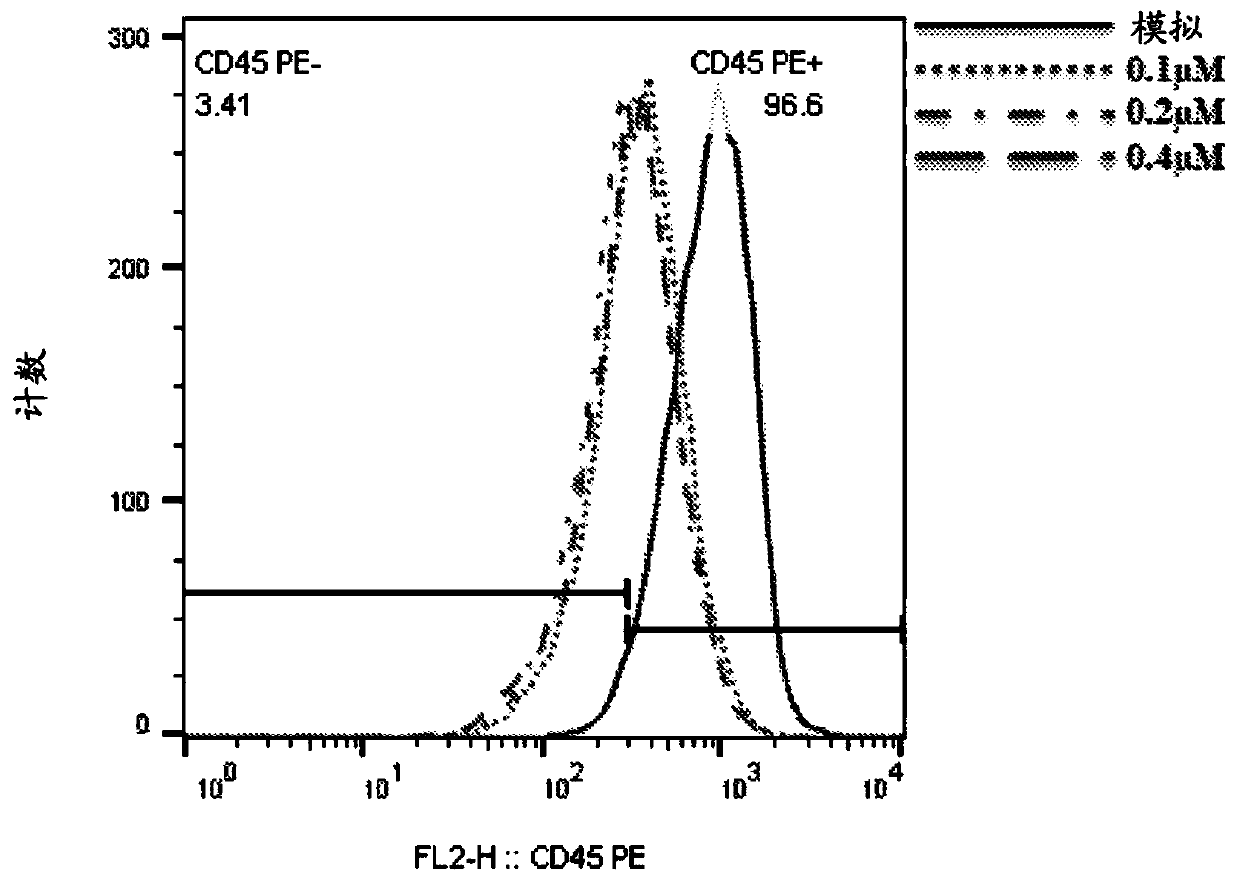
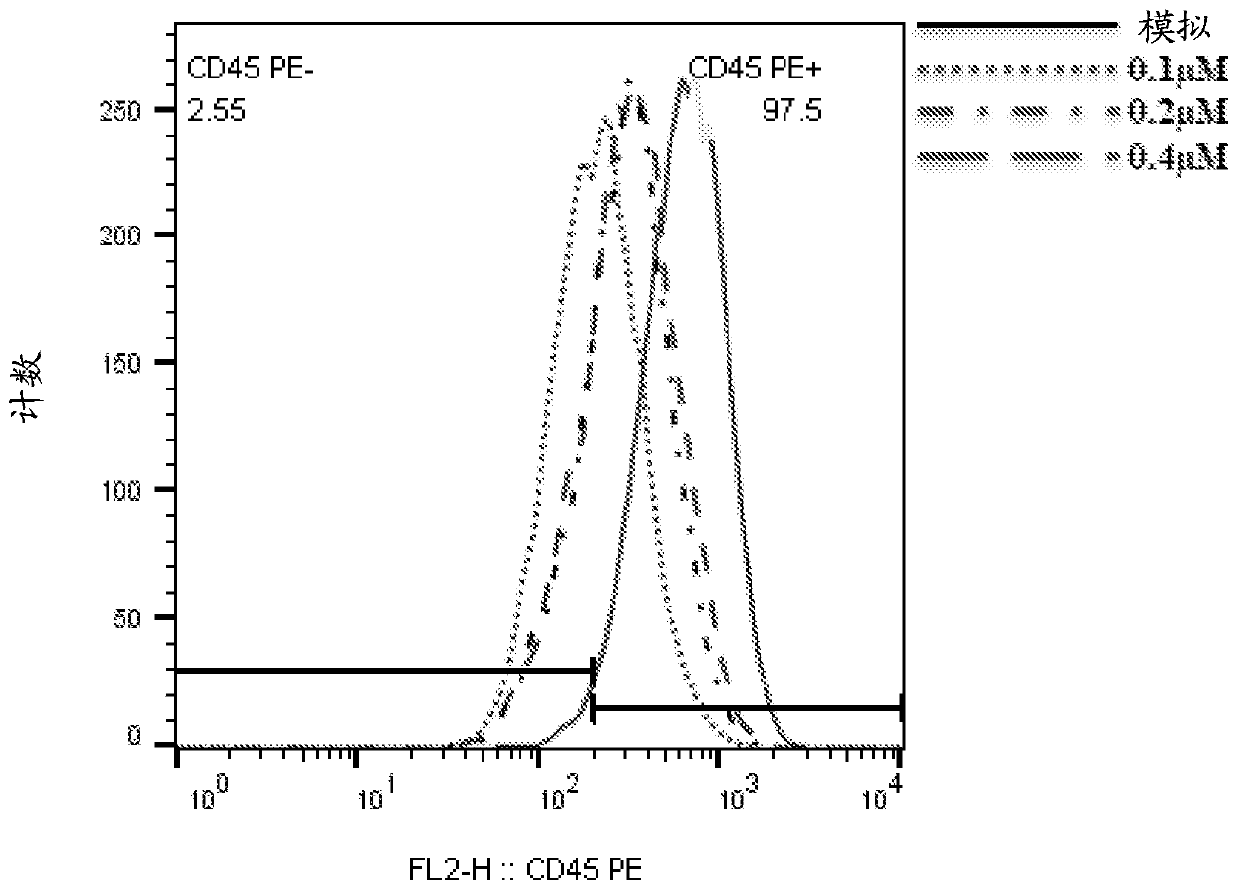
[0435] Monoclonal antibodies: anti-CD45Af647, Annexin-647 were purchased from BioLegend. Propidium iodide was purchased from Sigma-Aldrich.
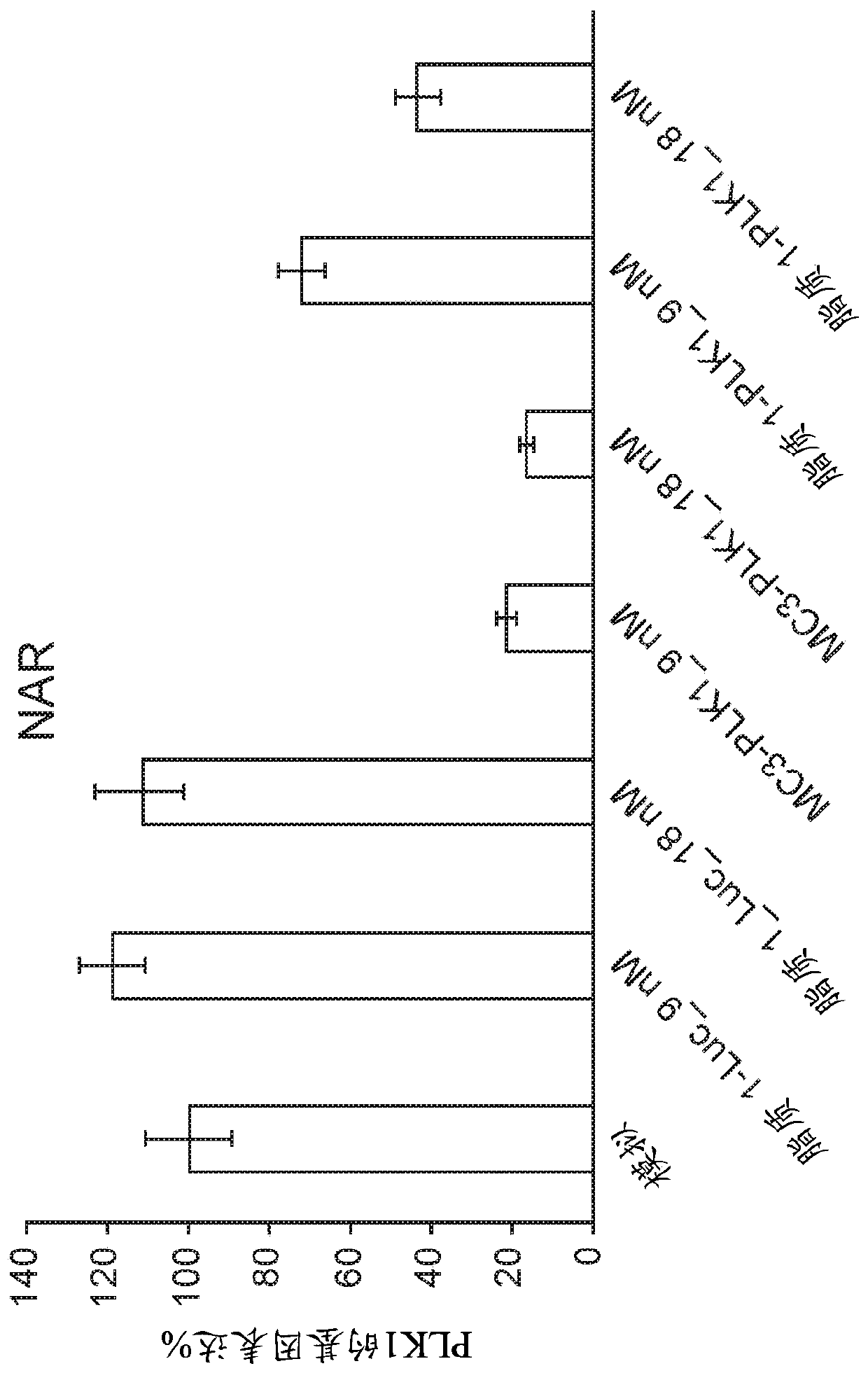
[0436] siRNA molecules were designed and screened by Alnylam Pharmaceuticals (USA).
[0437] Chemically modified siRNA sequences:
[0438] CD45 siRNA:
[0439] Sense strand: cuGGcuGAAuuucAGAGcAdTsdT (SEQ ID NO: 1)
[0440] Antisense strand: UGCUCUGAAAUUcAGCcAGdTsdT (SEQ ID NO: 2)
[0441] NC5 siRNA (siNC5 or ctl siRNA):
[0442] Sense strand: CAUAUUGCGCGUAUAGUCGCGUUAG
[0443] Antisense strand: UGGUAUAACGCGCAUAUCAGCGCAAUC
[0444] Luc siRNA (siLuc):
[0445] Sense strand: cuuAcGcuGAGuAcuucGAdTsdT (SEQ ID NO: 3)
[0446] Antisense strand: UCGAAGuACUcAGCGuAAGdTsdT (SEQ ID NO: 4)
[0447] Alexa-647-tagged siRNA has the sa...
Embodiment 2
[0470] Embodiment 2: the synthesis of lipid:
[0471] General Preparation (Illustrative Embodiments of Schemes 1-3).
[0472]
[0473]
[0474]
[0475] Method A:
[0476]Compound (i) or an amine functional compound as defined above is conjugated to a fatty acid via standard EDC / NHC coupling methods to give compound (ii). Further reduction with lithium aluminum hydride (LAH) followed by purification by column chromatography on silica gel gave the desired final compound (iii).
[0477] Method B:
[0478] Compound (i) and / or the amine functional compound and the corresponding aldehyde of the alkyl / alkenyl aliphatic chain were stirred at room temperature under argon for 2 hours, followed by NaCNBH 4 or sodium triacetoxyborohydride, and purified by silica gel column chromatography to give the desired final compound.
[0479] Method C:
[0480] Compound (i) or an amine functional compound as defined above is heated overnight with the corresponding alkyl / alkenyl brom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com