Synthesis method of cefotaxime acid
A technology of cefotaxamic acid and a synthesis method, applied in the field of medicine and chemical industry, can solve the problems of difficult recovery of dichloromethane and high cost, and achieve the effects of improving product conversion rate and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
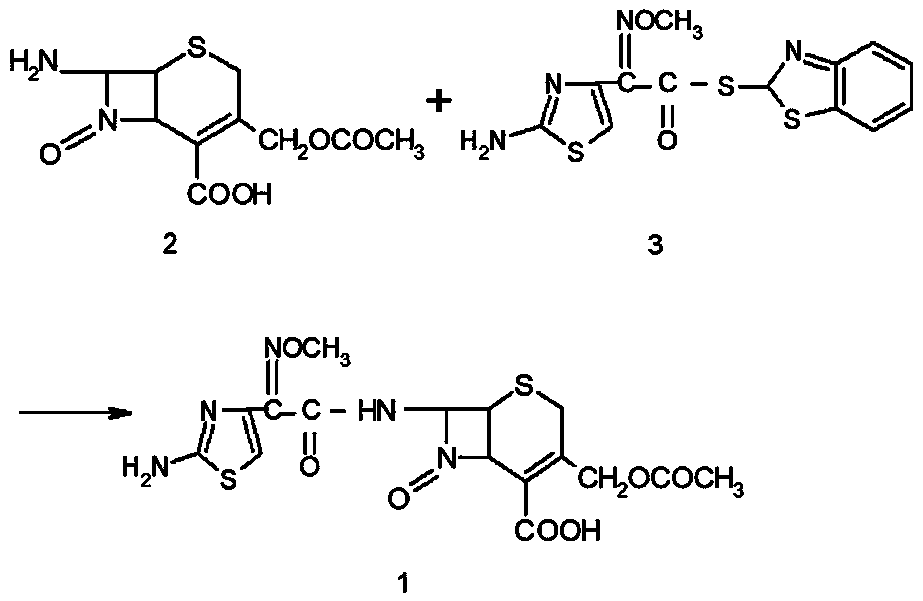
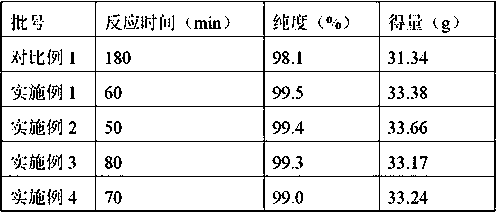
[0021] Mix 20.0g of 7-ACA and 26.0g of AE-active ester in a mixed solvent of 100mL of dichloromethane (moisture content: 0.17%) and 20mL of ethanol, add 4mL of purified water and 17mL of triethylamine, and react for 60min. Extract the organic phase twice with water, decolorize, adjust the pH=2.5-3.0 with hydrochloric acid, grow crystals for 1 hour, filter with suction to obtain 37g of cefotaxime acid wet powder, and dry to obtain 33.38g of cefotaxime acid. The purity is 99.5% after testing. , the water content is 0.6%, as shown in Table 1.
Embodiment 2
[0025] Mix 20.0g of 7-ACA and 26.0g of AE-active ester in a mixed solvent composed of 100mL of aqueous dichloromethane (moisture: 0.18%) and 20mL of ethanol, add 4mL of purified water and 17mL of triethylamine, and react with water for 50min. Extract the organic phase twice, decolorize, adjust the pH=2.5-3.0 with hydrochloric acid, grow crystals for 1 hour, filter with suction to obtain 37g of wet cefotaxime acid powder, and dry to obtain 33.66g of cefotaxime acid with a purity of 99.4% and a moisture content of 0.6%. See Table 1.
Embodiment 3
[0027] Mix 20.0g of 7-ACA and 26.0g of AE-active ester in a mixed solvent composed of 100mL of aqueous dichloromethane (moisture: 0.18%) and 20mL of DMF, add 3mL of purified water and 17mL of triethylamine, and react with water for 80min. Extract the organic phase twice, decolorize, adjust pH=2.5-3.0 with hydrochloric acid, grow crystals for 1 hour, filter with suction to obtain 37 g of wet cefotaxime acid powder, and dry to obtain 33.17 g of cefotaxime acid with a purity of 99.3% and a moisture content of 0.7%. See Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com