Preparation method of liraglutide intermediate polypeptide by genetic recombination
A technology of liraglutide and genetic recombination, which is applied in the field of biomedicine, can solve the problems of difficult recycling of waste liquid, long production cycle, and low renaturation efficiency, so as to reduce production costs and pollutants, and facilitate scale The effect of streamlining production and simplifying the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0033] Implementation Case 1 Construction of Recombinant Expression Vector
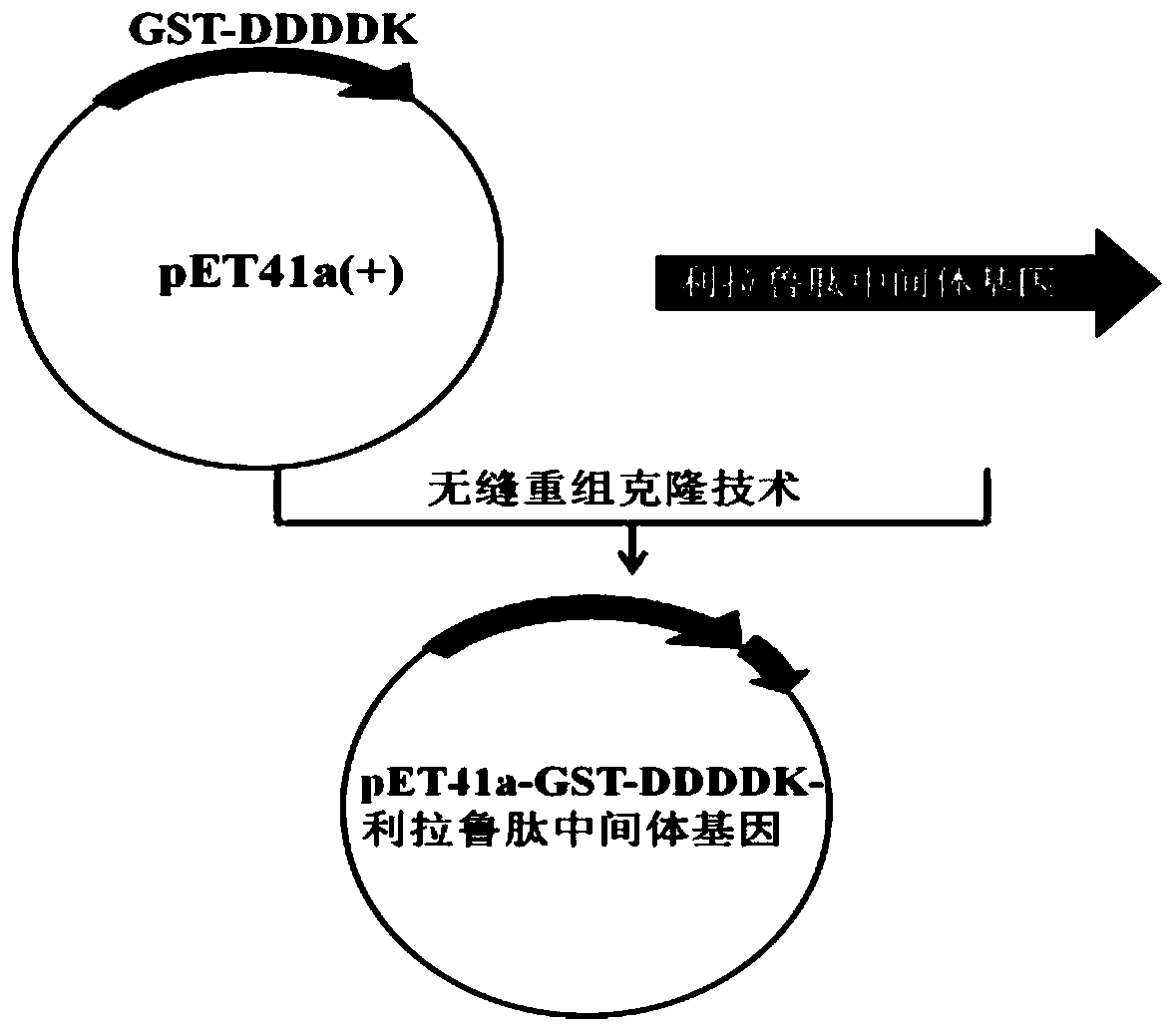
[0034] According to the codon preference of E. coli and other gene sequence factors related to E. coli expression, such as avoiding some cis-acting elements, the liraglutide intermediate gene sequence was optimized and synthesized. Then design primers for the liraglutide intermediate gene containing homology arms, clone and amplify it, and use a one-step cloning kit to directional clone it into the vector pET-41a(+) after the DDDDK coding sequence and before the terminator sequence, That is, the recombinant expression vector pET-41a-GST-DDDDK-liraglutide intermediate was constructed as figure 1 shown.
Embodiment example 2
[0035] Construction of implementation case 2 recombinant expression strain
[0036] The obtained recombinant expression vector was transformed into the expression host strain Escherichia coli BL21 (DE3) according to the conventional heat shock transformation method.
Embodiment example 3
[0037] Implementation Case 3 Expression and Purification of Recombinant Proteins
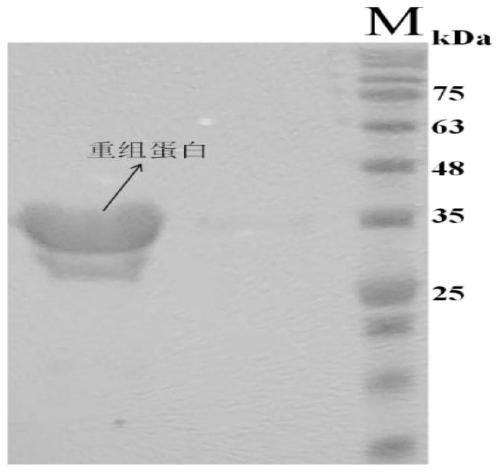
[0038] The obtained recombinant strain was inoculated into LB medium containing ampicillin at a final concentration of 50ug / ml and cultured in shake flasks for 5 hours, and then added with IPTG at a final concentration of 50ug / ml for induction for 4 hours to allow sufficient expression of the recombinant protein. The cells were collected by centrifugation, resuspended in PBS buffer, and disrupted by ultrasonication. The supernatant was collected by centrifugation, and the recombinant protein was affinity-adsorbed through a GST purification column. Then, the recombinant protein (about 35kDa) was eluted with Tris-HCl buffer containing 20mmol / L reduced glutathione, such as figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com