Normal-phase chromatography analysis method for 4-chloro-2-nitrophenyl isocyanate and reaction liquid thereof
A technology of nitrophenyl isocyanate and normal phase chromatography, which is applied in the field of normal phase chromatography analysis, can solve the problem that 4-chloro-2 nitrophenyl isocyanate cannot be analyzed at the same time, and achieve the effect of rapid method and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Direct quantitative analysis of 4-chloro-2-nitrophenyl isocyanate
[0044] 1. Chromatographic separation and detection conditions
[0045] Chromatographic column: Agilent's normal-phase silica gel column RX-SIL (diameter and length: 4.6×100 mm, silica gel particle diameter: 1.8 μm).
[0046] Mobile phase: 0.10% trifluoroacetic acid ~ 99.90% n-hexane, total water content <0.01%.
[0047] Flow rate: 1.0ml / min
[0048] Column temperature: 25°C
[0049] Detection wavelength of UV detector: 230nm
[0050] Injection volume: 1.0μL
[0051] 2. Preparation of standard solution and sample solution
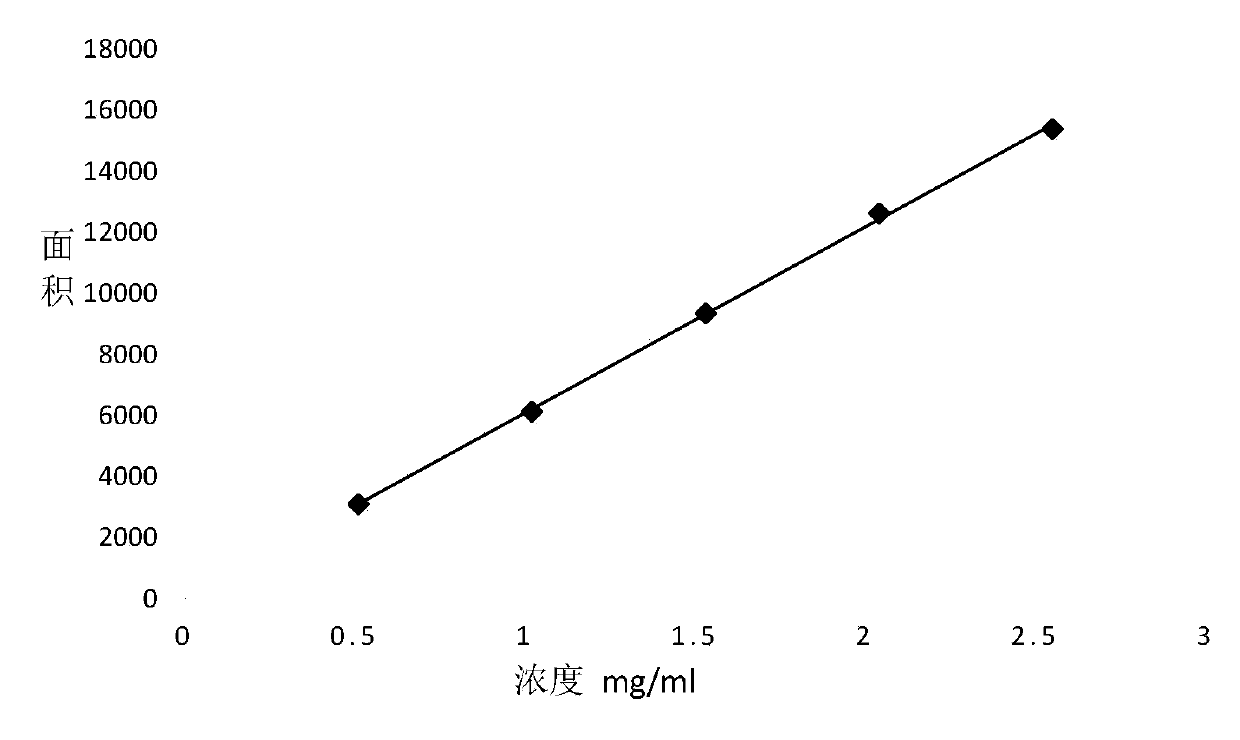
[0052] Accurately weigh 30mg, 60mg, 90mg, 120mg and 150mg (accurate to 0.0001g) of self-made standard samples of 4-chloro-2-nitrophenylisocyanate into a 10ml brown volumetric flask, add 0.10 % trifluoroacetic acid ~ 99.90% n-hexane dissolved and constant volume to obtain standard samples 1 ~ 5.
[0053]Accurately weigh a sample of 100 mg (accurate to 0.0001 g) of 4-chloro-2-nit...
Embodiment 2
[0067] The Intermediate Control Analysis of the Reaction Solution Synthesized from 4-Chloro-2-Nitroaniline and Phosgene at Normal Temperature
[0068] Chromatographic separation and detection conditions are the same as in Example 1.
[0069] Accurately weigh 0.02g (accurate to 0.0001g) of 4-chloro-2nitroaniline into a 25ml brown volumetric flask, add 0.10% trifluoroacetic acid-99.90% n-hexane with a total water content of image 3 .
[0070] Accurately pipette 0.2 g of the reaction solution into a 25 ml brown volumetric flask, add 0.10% trifluoroacetic acid-99.90% n-hexane with a total water content of <0.01% to dissolve and dilute to volume; obtain the sample to be analyzed.
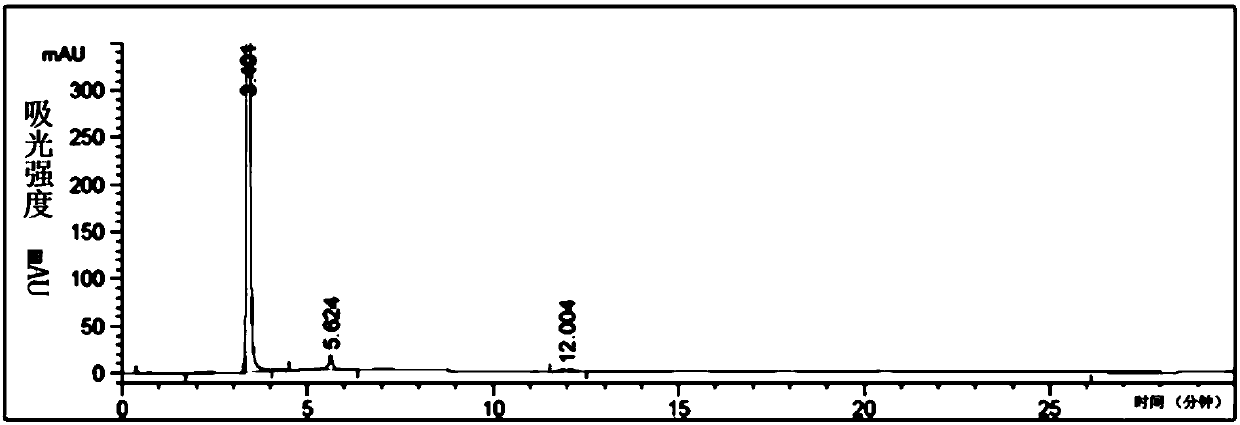
[0071] The chromatographic analysis chart of the reaction solution under normal temperature conditions is shown in Figure 4 .
[0072] Under selected chromatographic conditions, using the above-mentioned analysis method, the external standard method can qualitatively determine whether there are raw m...
Embodiment 3
[0074] The intermediate control analysis of the reaction liquid synthesized from 4-chloro-2-nitroaniline and phosgene at 50℃ for the synthesis of 4-chloro-2-nitrophenyl isocyanate.
[0075] Chromatographic separation and detection conditions are the same as in Example 1.
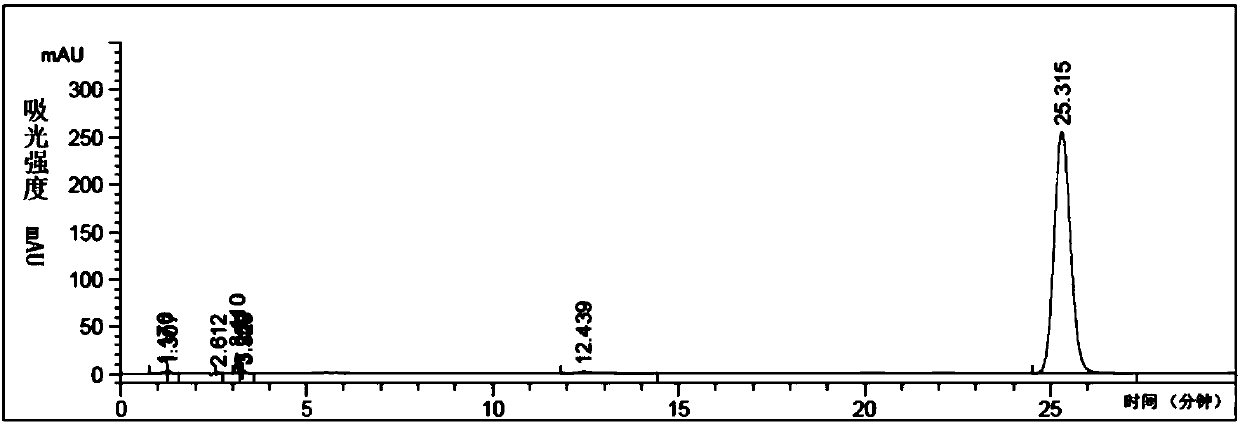
[0076] Sampling analysis of the reaction solution sample configured in Example 2, the chromatographic analysis spectrum of the reaction solution at 50°C is shown in Figure 5 .
[0077] Under the selected chromatographic conditions, using the above analysis method, the external standard method can qualitatively determine whether there are raw materials, intermediate products and products in the reaction solution at 50°C. By using the area normalization method to relative quantify the by-products, when the main product 4-chloro-2-nitrophenyl isocyanate has the highest area percentage content, the optimal synthesis reaction conditions at 50°C are determined accordingly.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com