Kits for the detection of respiratory pathogens in community-acquired pneumonia
A technology for acquired pneumonia and respiratory tract, which is applied in the field of biomedical detection and can solve problems such as complex pathogen spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
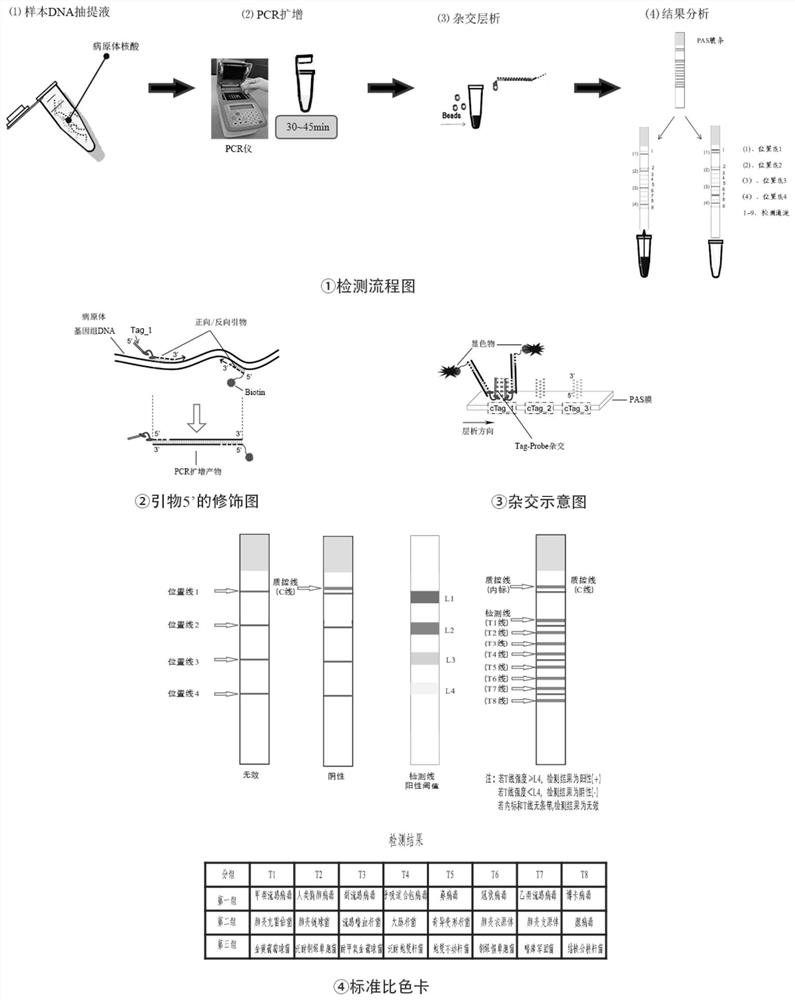
[0090] This embodiment provides a kit for detecting respiratory pathogens of community-acquired pneumonia, which includes a reaction solution, a primer solution, an enzyme solution, a negative quality control, a positive quality control, a hybridization chromogenic solution, a hybridization membrane strip, and Desiccant; reaction solution includes PCR buffer solution, metal cations and purified water, enzyme solution includes reverse transcriptase, hot start Taq enzyme and UNG enzyme, negative quality control product is sterile normal saline, positive quality control product is for the purpose of detecting pathogens The cloning bacteria liquid or pseudovirus particles of the amplified sequence, the base sequence of the amplified sequence of the pathogen target is shown in SEQ ID NO.60-83; the concentration of metal cations is 1-3mM, and the concentration of Taq enzyme is 0.5-5U, The concentration of UNG enzyme is 0.05~0.5U, the concentration of cloning bacteria is 1×10 4 cfu / m...
Embodiment 2
[0097] Specifically, using the detection kit described in the above-mentioned embodiment 1, this embodiment shows the detection data of three groups of clinical samples. Clinical samples are nasopharyngeal swabs, alveolar lavage fluid or sputum samples from CAP patients with known pathogen infection (see Table 7: " / " in Table 7 indicates no clinically confirmed infection pathogen type, and these samples are negative ). The pretreatment of clinical samples is as follows: (1) nasopharyngeal swab sample: put the swab sample into 1.0ml of normal saline, wash it thoroughly, squeeze it dry, discard the swab, centrifuge at 12000rpm for 5min, discard the supernatant, and leave the precipitate Standby; (2) Alveolar lavage fluid sample: take 1.0ml sample, centrifuge at 12000rpm for 5min, discard the supernatant, then add 1.0ml of normal saline to wash once, centrifuge at 12000rpm for 5min, discard the supernatant, and save the precipitate for later use; (3) Sputum sample: a. Add 2 to 3...
Embodiment 3
[0115] In order to prove the rationality and feasibility of the present invention, the following performance evaluation research has been carried out to the detection kit:
[0116] (1) Minimum detection limit experiment
[0117] Preparation of reference products: purchase standard reference products from the Guangdong Microbial Culture Collection Center (see the table below), extract the nucleic acid of the reference product, measure the nucleic acid concentration, convert the copy number according to the relevant formula, and then use 0.2×TE to test the nucleic acid sample Serial dilution and detection.
[0118] Reference Detection concentration gradient (copies / ml) SA 10 4 、10 3 、500、100
PA 10 4 、10 3 、500、100
IFB 10 4 、10 3 、500、100
[0119] The standard reference substance of each concentration gradient is detected by the detection method of the detection kit in the above-mentioned embodiment, and the membrane strip detection resu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com