Compound tetracaine hydrochloride membrane and preparation method thereof
A tetracaine hydrochloride film and the technology of tetracaine hydrochloride are applied in the directions of pharmaceutical formulations, medical preparations with inactive ingredients, medical preparations containing active ingredients, etc. The effect of uniform texture and improved production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of embodiment 1 compound tetracaine hydrochloride film
[0028] Include the following steps:
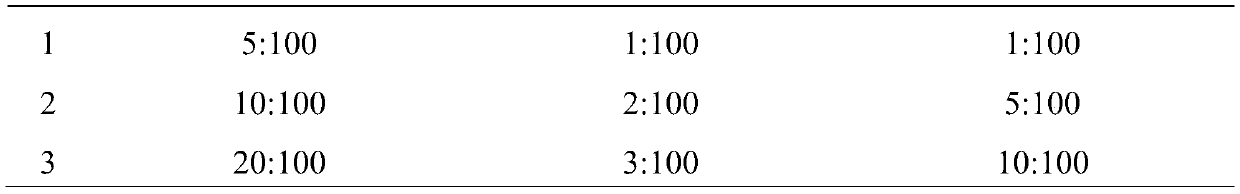
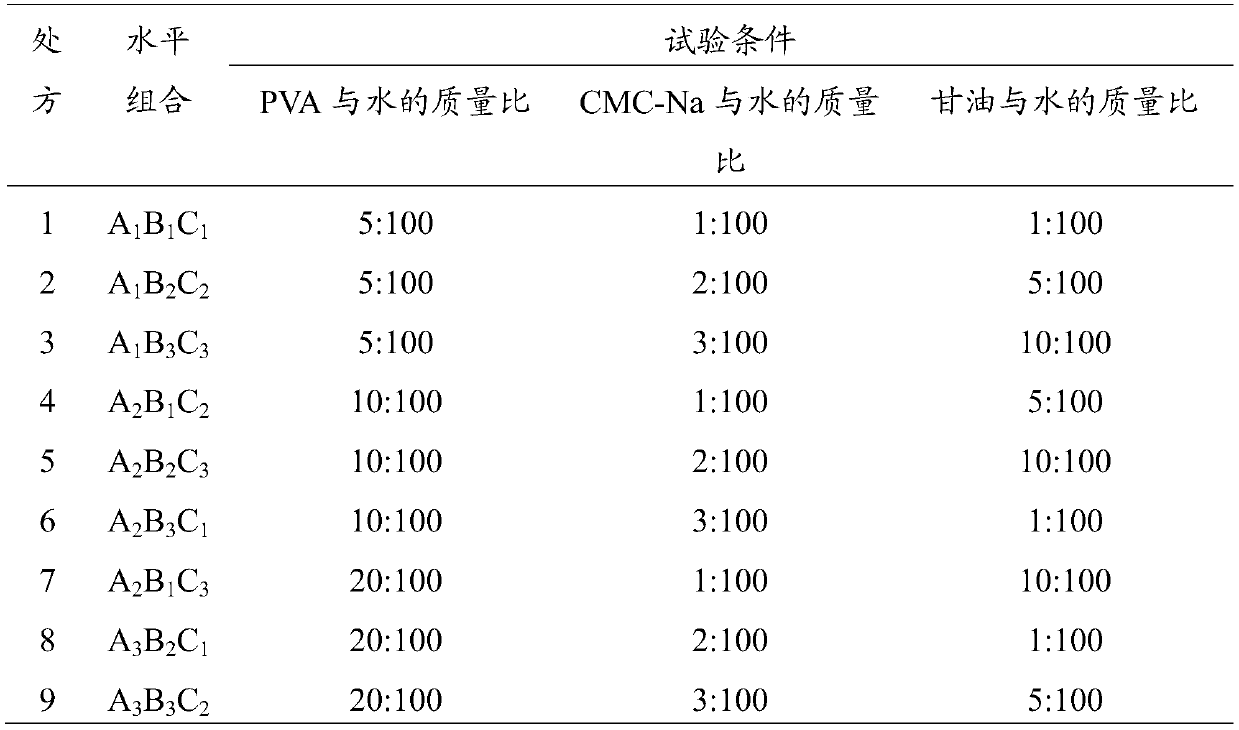
[0029] (1) Take tetracaine hydrochloride, gentamycin sulfate, hydrocortisone, carboxymethylcellulose sodium (CMC-Na), polyvinyl alcohol (PVA), glycerol respectively according to the parts by weight described below and purified water:
[0030] 0.4 parts by weight of tetracaine hydrochloride, 0.2 parts by weight of gentamicin sulfate, 0.2 parts by weight of hydrocortisone, 1.6 parts by weight of sodium carboxymethylcellulose (CMC-Na), 8 parts by weight of polyvinyl alcohol (PVA), 4 parts by weight of glycerin, 80 parts by weight of purified water.
[0031] (2) Add the weighed carboxymethylcellulose sodium (CMC-Na) and polyvinyl alcohol (PVA), add appropriate amount of purified water to soak and swell fully, heat and dissolve on a water bath at 85-90°C, avoid stirring, and make it Naturally dissolve completely to obtain film slurry, cool for later use;
[0032...
Embodiment 2
[0035] The preparation of embodiment 2 compound tetracaine hydrochloride film
[0036] Include the following steps:
[0037] (1) Take tetracaine hydrochloride, gentamycin sulfate, hydrocortisone, carboxymethylcellulose sodium (CMC-Na), polyvinyl alcohol (PVA), glycerol respectively according to the parts by weight described below and purified water:
[0038] 0.5 parts by weight of tetracaine hydrochloride, 0.2 parts by weight of gentamicin sulfate, 0.1 parts by weight of hydrocortisone, 1 part by weight of sodium carboxymethylcellulose (CMC-Na), 15 parts by weight of polyvinyl alcohol (PVA), 12 parts by weight of glycerin, and 90 parts by weight of purified water.
[0039] (2) Add the weighed carboxymethylcellulose sodium (CMC-Na) and polyvinyl alcohol (PVA), add appropriate amount of purified water to soak and swell fully, heat and dissolve on a water bath at 85-90°C, avoid stirring, and make it Naturally dissolve completely to obtain film slurry, cool for later use;
[0...
Embodiment 3
[0043] The preparation of embodiment 3 compound tetracaine hydrochloride film
[0044] Include the following steps:
[0045] (1) Take tetracaine hydrochloride, gentamycin sulfate, hydrocortisone, carboxymethylcellulose sodium (CMC-Na), polyvinyl alcohol (PVA), glycerol respectively according to the parts by weight described below and purified water:
[0046] 0.3 parts by weight of tetracaine hydrochloride, 0.3 parts by weight of gentamycin sulfate, 0.3 parts by weight of hydrocortisone, 2 parts by weight of sodium carboxymethylcellulose (CMC-Na), 5 parts by weight of polyvinyl alcohol (PVA), 4 parts by weight of glycerin, 70 parts by weight of purified water.
[0047] (2) Add the weighed carboxymethylcellulose sodium (CMC-Na) and polyvinyl alcohol (PVA), add appropriate amount of purified water to soak and swell fully, heat and dissolve on a water bath at 85-90°C, avoid stirring, and make it Naturally dissolve completely to obtain film slurry, cool for later use;
[0048] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com