Multi-metal MOFs derived oxygen reduction/oxygen evolution double-function catalytic material, and preparation method thereof
A dual-functional catalysis, multi-metal technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve the problems of complex preparation process and unstable catalytic activity, increase specific surface area, promote Electron transfer during adsorption and desorption and catalysis, high porosity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
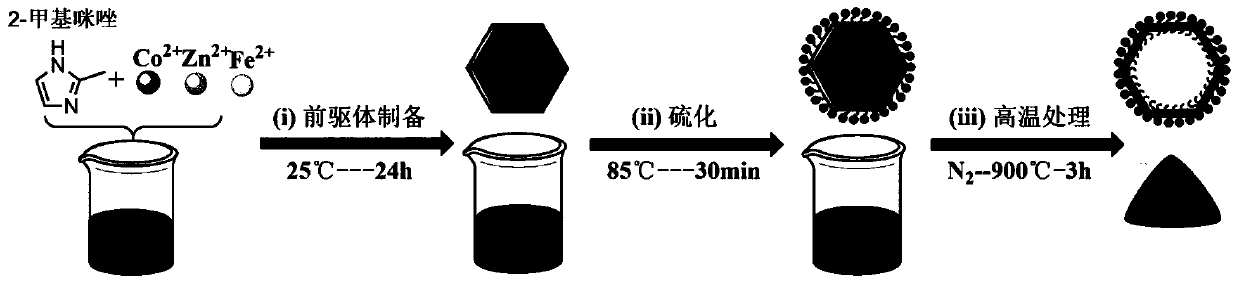
[0032] Such as figure 1 As shown, a method for preparing an oxygen reduction / oxygen evolution bifunctional catalytic material derived from multimetal MOFs, the steps are as follows:
[0033] (1) Preparation of the precursor: First, weigh 9.6 mmol of Zn(NO 3 ) 2 ·6H 2 O, 2.4mmol of Co(NO 3 ) 2 ·6H 2 O and 0.24mmol of FeSO 4 ·7H 2The O mixture was dissolved in 125 mL of methanol, and stirred evenly to obtain solution A. Next, weigh 48mmol of 2-methylimidazole and dissolve it in 45mL of methanol, and stir evenly to obtain solution B. Then solution A was slowly poured into solution B, the mixture was stirred at room temperature for 25 minutes, and the precursor product could be obtained after 24 hours of co-precipitation reaction at room temperature. Finally, the obtained product was centrifuged and washed three times with absolute ethanol and deionized water respectively, and dried in a vacuum oven at 60°C for 24 hours, recorded as Zn 0.8 co 0.2 Fe 0.02 -ZIF (Zn / Co / Fe...
Embodiment 2
[0039] Such as figure 1 As shown, a method for preparing an oxygen reduction / oxygen evolution bifunctional catalytic material derived from multimetal MOFs, the steps are as follows:
[0040] (1) Preparation of the precursor: First, weigh 7.2 mmol of Zn(NO 3 ) 2 ·6H 2 O, 4.8mmol of Co(NO 3 ) 2 ·6H 2 O and 0.48mmol of FeSO 4· 7H 2 The O mixture was dissolved in 125 mL of methanol, and stirred evenly to obtain solution A. Next, weigh 48mmol of 2-methylimidazole and dissolve it in 45mL of methanol, and stir evenly to obtain solution B. Then solution A was slowly poured into solution B, the mixture was stirred at room temperature for 25 minutes, and the precursor product could be obtained after 24 hours of co-precipitation reaction at room temperature. Finally, the obtained product was centrifuged and washed three times with absolute ethanol and deionized water respectively, and dried in a vacuum oven at 60°C for 24 hours, recorded as Zn 0.6 co 0.4 Fe 0.04 -ZIF (Zn / Co / F...
Embodiment 3
[0046] Such as figure 1 As shown, a method for preparing an oxygen reduction / oxygen evolution bifunctional catalytic material derived from multimetal MOFs, the steps are as follows:
[0047] (1) Preparation of the precursor: First, weigh 6.0 mmol of Zn(NO 3 ) 2 ·6H 2 O, 6.0mmol of Co(NO 3 ) 2 ·6H 2 O and 0.6mmol of FeSO 4 ·7H 2 The O mixture was dissolved in 125 mL of methanol, and stirred evenly to obtain solution A. Next, weigh 48mmol of 2-methylimidazole and dissolve it in 45mL of methanol, and stir evenly to obtain solution B. Then solution A was slowly poured into solution B, the mixture was stirred at room temperature for 25 minutes, and the precursor product could be obtained after 24 hours of co-precipitation reaction at room temperature. Finally, the obtained product was centrifuged and washed three times with absolute ethanol and deionized water respectively, and dried in a vacuum oven at 60°C for 24 hours, recorded as Zn 0.5 co 0.5 Fe 0.05 -ZIF (Zn / Co / Fe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com