Synthesis method and agricultural biological activity of benzothiadiazines
A technology of benzothiadiazine and synthesis method, applied in the directions of chemicals, biocides, organic chemistry, etc. for biological control, can solve the problems of uneconomical, expensive amount of metal rhodium catalyst, and high experimental cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
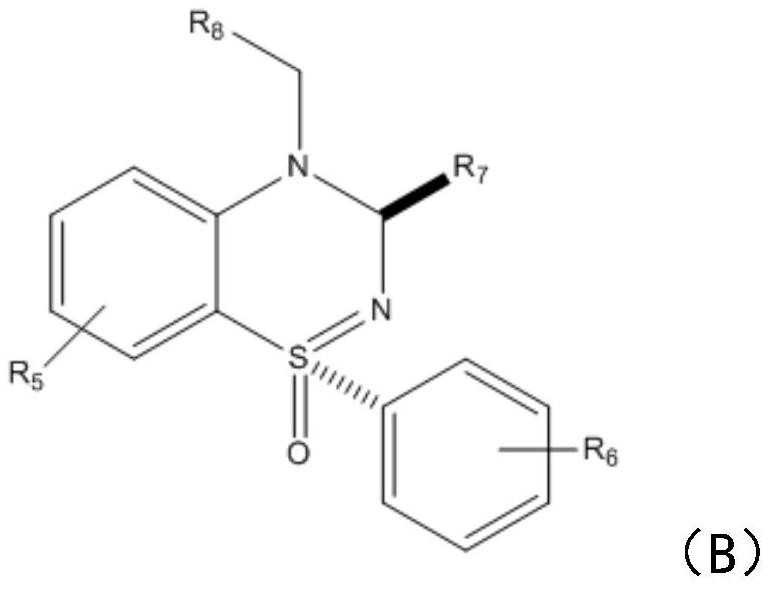
[0021] The embodiment of the present invention provides a synthesis method of benzothiadiazine compounds, comprising the following steps:
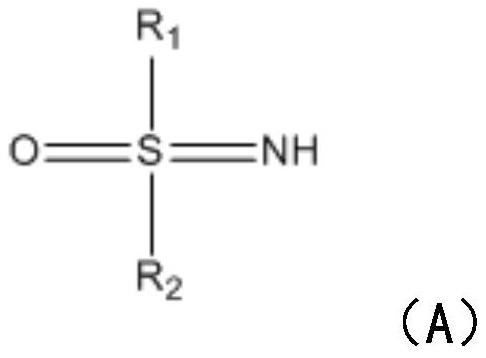
[0022] S1: Add sulfinimide compounds and nitrobenzene compounds to the reactor respectively, under the action of nickel diacetylacetonate or nickel chloride and solvent, seal and react at 80-110°C for 5-8 hours in air .
[0023] In this step, benzothiadiazine compounds are synthesized by using sulfinimide compounds and nitrobenzene compounds. Specifically, under the action of catalyst nickel, sulfenimide compounds react with nickel to form five The membered ring compound is then further reacted with nitrobenzene compounds to introduce amino groups, and finally continues to react with another molecule of nitrobenzene compounds to generate the target product. What needs to be explained here is that this step reaction does not need to be heated, and the reaction can be carried out under air conditions. The catalyst dosage of nickel diacetyla...
Embodiment 1
[0040] Add 1mmol to the reactor 2.3mmol 0.02mmol of nickel diacetylacetonate and 3ml of 1,1-dichloroethane were sealed and reacted at 100°C in air for 5 hours; after the reaction, column chromatography was performed to obtain the following C1 compound:
[0041]
[0042] Carry out nuclear magnetic spectrum analysis to above-mentioned light yellow solid powder, data is as follows:
[0043] 1 H NMR (500MHz, CDCl 3 )δ=8.29(d, J=8.4Hz, 2H), 8.17(d, J=8.4Hz, 2H), 7.95(d, J=7.6Hz, 2H), 7.67-7.62(m, 5H), 7.53( t,J=7.6Hz,2H),7.37-7.31(m,2H),6.83(d,J=8.5Hz,1H),6.72(t,J=7.6Hz,1H),6.21(s,1H), 5.16(d, J=17.6Hz, 1H), 4.61(d, J=17.1Hz, 1H);
[0044] 13 C NMR (125MHz, CDCl 3)δ=148.8, 147.7, 147.4, 144.3, 144.3, 140.2, 134.8, 133.4, 129.2, 128.6, 128.4, 127.8, 127.6, 124.4, 124.0, 121.6, 118.3, 114.7, 75.9, 53.8;
[0045] After identification, the spectral data corresponded to the structural formula, proving that the synthesized product was (1S,3R)-4-(4-nitrobenzyl)-3-(4-nitrophen...
Embodiment 2
[0047] Add 1mmol to the reactor 2.3mmol 0.02mmol of nickel chloride and 3ml of 1,1-dichloroethane were sealed and reacted at 110°C for 8 hours in the air; after the reaction, column chromatography was performed to obtain the following C2 compound:
[0048]
[0049] Carry out nuclear magnetic spectrum analysis to above-mentioned light yellow solid powder, data is as follows:
[0050] 1 H NMR (500MHz, CDCl 3 )δ=8.26(d, J=8.4Hz, 2H), 8.17(d, J=8.8Hz, 2H), 7.83(d, J=8.0Hz, 2H), 7.66(d, J=8.4Hz, 2H) ,7.61(d,J=8.4Hz,2H),7.32(t,J=8.0Hz,2H),7.21(d,J=8.0Hz,1H),6.63(s,1H),6.54(d,J= 8.0Hz, 1H), 6.21(s, 1H), 5.11(d, J=17.2Hz, 1H), 4.58(d, J=17.6Hz, 1H), 2.43(s, 3H), 2.25(s, 3H) ;
[0051] 13 C NMR (125MHz, CDCl 3 )δ=149.1, 147.7, 147.5, 145.9, 144.6, 144.4, 144.2, 137.6, 130.1, 128.6, 128.3, 127.8, 127.6, 124.4, 123.9, 119.6, 119.4, 114.8, 75.8, 53.8, 21.4, 262.4;
[0052] After identification, the spectral data corresponded to the structural formula, proving that (1S,3R)-6-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com