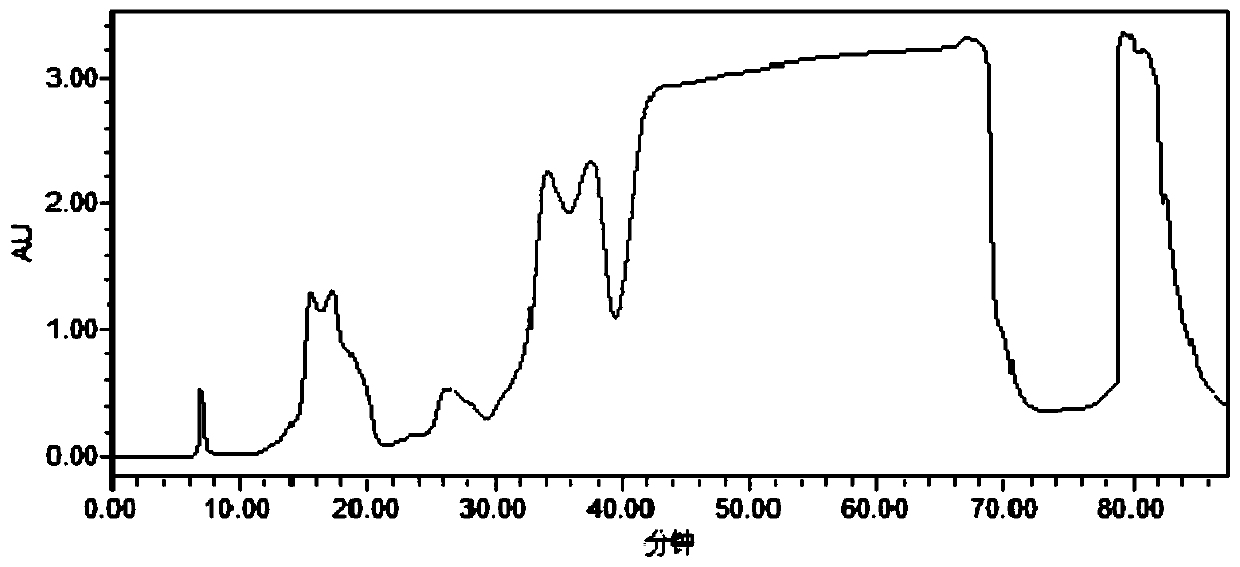
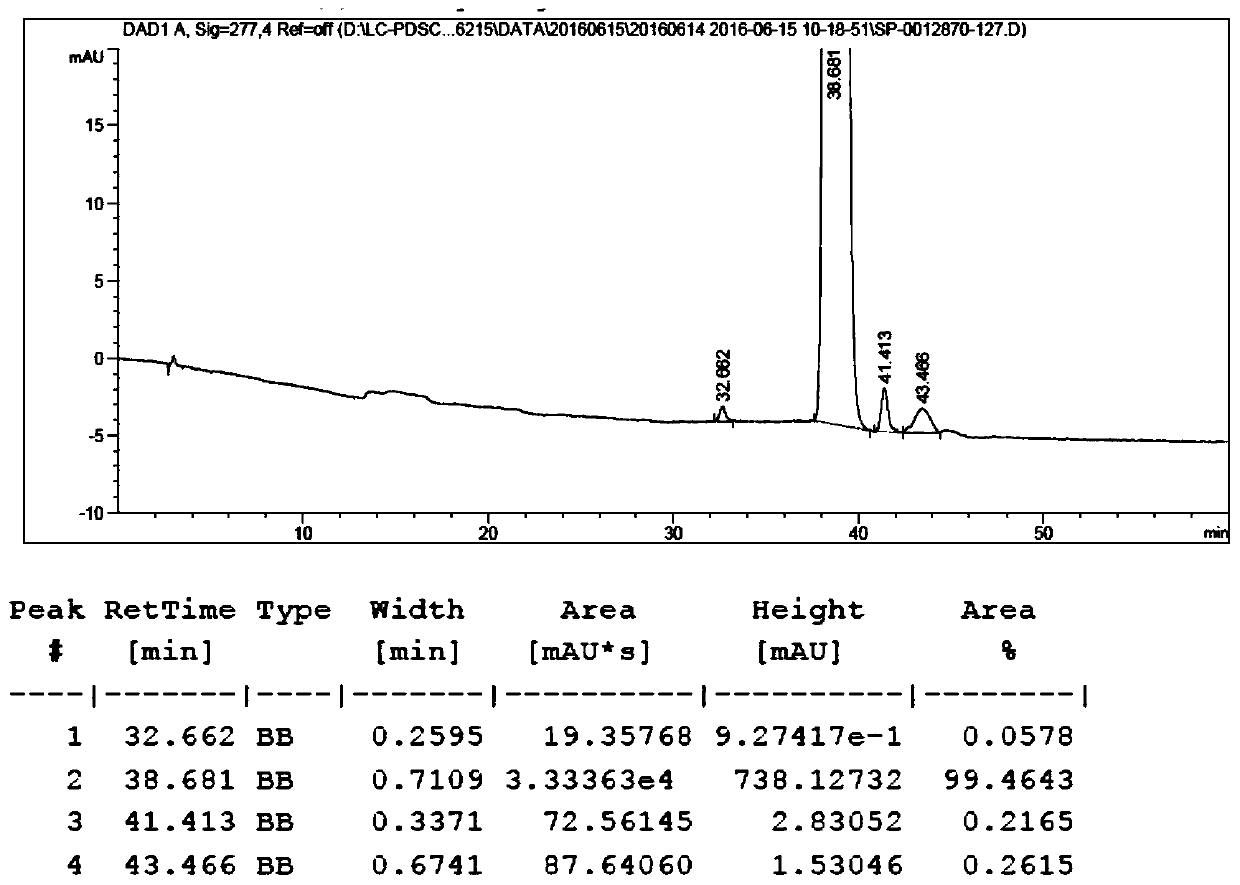
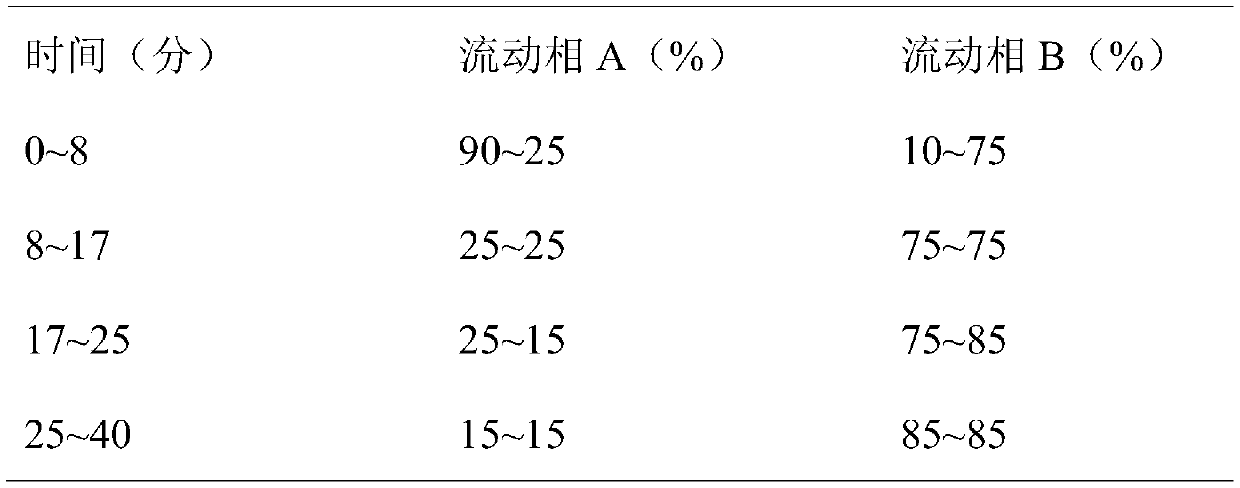
Method for separating and purifying temsirolimus
A technology for separation and purification of temsirolimus, applied in the field of separation and purification of generic drugs, can solve the problems of inflammable and explosive, unsuitable for industrialized large-scale production, low boiling point of diethyl ether, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 Separation and purification of temsirolimus of the present invention
[0027] a. Dissolve 2,2,5-trimethyl-5-carboxylic acid-1,3-dioxane in n-hexane, add thionyl chloride at 25±5°C, mix the mass volume ratio m 2,2,5-三甲基-5-羧酸-1,3-二氧六烷 :V 正己烷 :m 二氯亚砜 =1g: 14ml: 1.37g, stirred for 15h. Concentrate to dryness under reduced pressure to obtain a colorless oily liquid, the acid chloride.
[0028] b. Mix rapamycin, N, N-diisopropylethylamine (DIPEA) with dichloromethane (DCM), its mass volume ratio m 雷帕霉素 :V DIPEA :V DCM =1g: 0.72ml: 2.83ml, drop the acid chloride prepared in the above a at 0-5°C, so that the concentration of the acid chloride is 1g / ml, stir and react at 25±5°C for 22h, then take a sample, and detect the content of rapamycin by HPLC After less than 0.5%, concentrated and evaporated to dryness, the obtained crude product was purified by 200-300 mesh column silica gel with petroleum ether: ethyl acetate 1:1 as eluent, and the esterified product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com