Preparation method of fusion gene positive control standard
A technology of fusion gene and positive control, applied in the field of biomedicine, can solve the problems of long production cycle, high cost, inflexible replacement of reference genes, etc., and achieve the effects of flexible use, cost saving, easy quantification and preservation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] A kind of preparation method of fusion gene positive control standard substance of the present invention comprises the following steps:
[0033] (1) extracting the total RNA in the expression sample of the fusion partner gene, and synthesizing cDNA by reverse transcription;
[0034] (2) Using the cDNA synthesized in step (1) as a template, and using gene-specific primers and gene junction primers as primers, amplify the upstream fusion partner gene and the downstream fusion partner gene of the fusion gene respectively by asymmetric PCR; wherein, for The molar concentration ratio of gene-specific primers and gene-junction primers for amplification of upstream fusion partner genes is 5-20:1, and the molar concentration ratio of gene-specific primers and gene-junction primers for amplification of downstream fusion partner genes is 5-20: 1;
[0035] (3) Mixing the PCR amplification products of the upstream fusion partner gene and the downstream fusion partner gene respecti...
Embodiment 1
[0051] Example 1 Non-small cell lung cancer ALK, preparation of ROS1 and RET fusion gene positive control standard
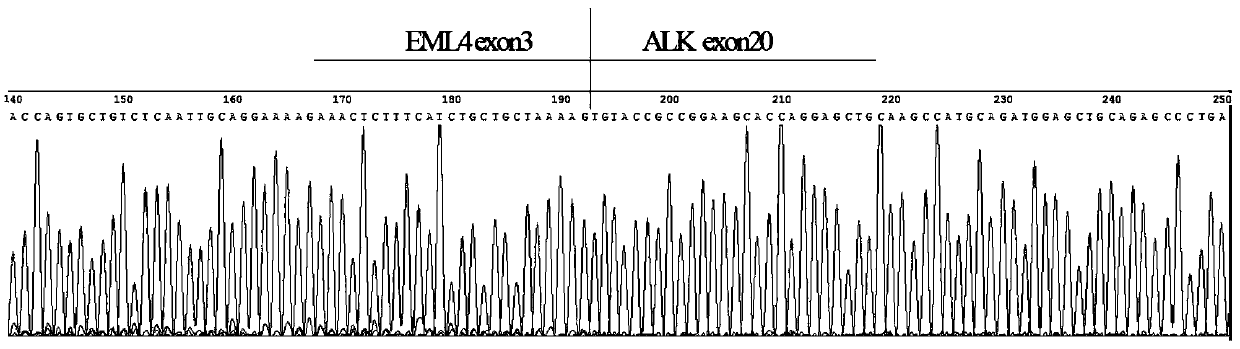
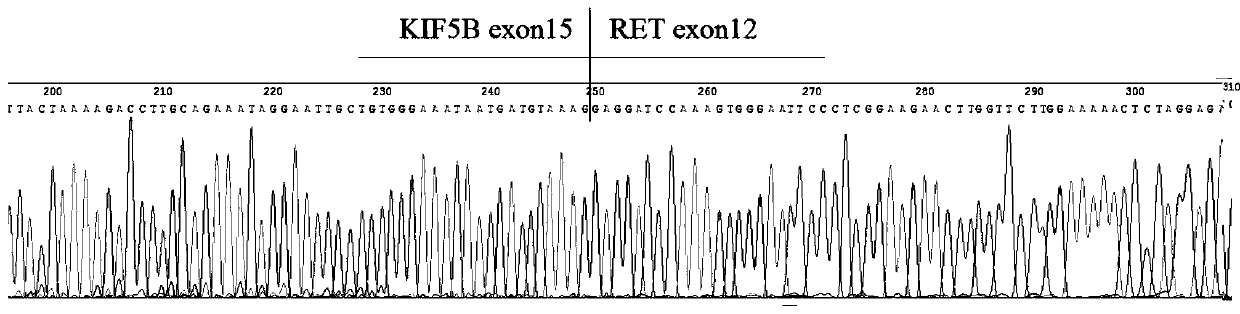
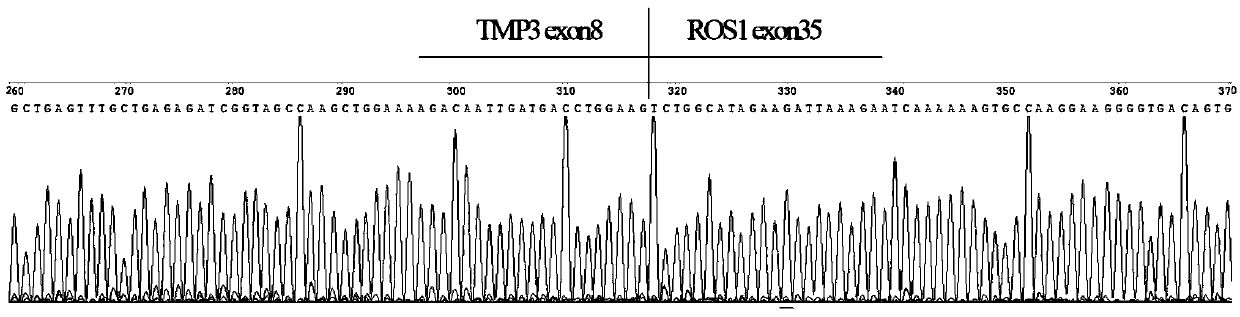
[0052] 1. According to the database (COSMIC v85), screen the ALK, ROS1 and RET fusion gene types and gene information reported in lung cancer, the fusion genes include: EML4(2)-ALK(20), EML4(3)-ALK(20 ), EML4(10)-ALK(20), EML4(14)-ALK(20), EML4(15)-ALK(20), EML4(17)-ALK(20), EML4(18)-ALK(20 ), EML4(20)-ALK(20), STRN(3)-ALK(20), TFG(4)-ALK(20), KLC1(9)-ALK(20), KLC1(10)-ALK(20 ), HIP1(21)-ALK(20), HIP1(28)-ALK(20), HIP1(30)-ALK(20), KIF5B(15)-ALK(20), KIF5B(17)-ALK(20 ), KIF5B(24)-ALK(20), CD74(6)-ROS1(32), CD74(6)-ROS1(34), EZR(10)-ROS1(34), TPM3(8)-ROS1(35 ), LRIG3(16)-ROS1(35), SLC34A2(4)-ROS1(32), SLC34A2(13)-ROS1(32), GOPC(4)-ROS1(36), SDC4(2)-ROS1(32 ), SDC4(2)-ROS1(34), SDC4(4)-ROS1(32), CCDC6(11)-RET(12), KIF5B(15)-RET(12), KIF5B(16)-RET(12 ), KIF5B(22)-RET(12), KIF5B(23)-RET(12), KIF5B(15)-RET(11), KIF5B(24)-RET(11).
[0053] Since the fusion genes ...
Embodiment 2
[0079] Example 2 Preparation and Application of EGFR Copy Number Analysis Internal Control Standard CFTR(7)-EGFR(20) Fusion Gene
[0080] 1. According to the literature, select the relatively conserved segment sequence of the CFTR gene on the same chromosome as the EGFR gene and on different chromosome arms as the reference gene for the analysis of the copy number of the EGFR gene. Using the HCT116 cell cDNA synthesized in Example 1 as a template, CFTR and EGFR gene fragments were amplified by asymmetric PCR respectively, and the primers used were SEQ ID NO:21~SEQ ID NO:22 and SEQ ID NO:95~SEQ ID NO:96 , amplification method, system, and conditions are the same as in Example 1.
[0081] 2. The self-annealing PCR reaction system and reaction conditions are the same as in Example 1.
[0082] 3. the self-annealing PCR product that obtains in above-mentioned steps 2 is diluted 1000 times and does template, and nested PCR amplifies the target fusion gene fragment, and primer seque...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com