Method for synthesizing aryl monofluoromethylthio compound from Bunte salt
A technology of methylthio compounds, applied in the field of organic synthesis, can solve the problems of unfavorable industrial production and difficult accurate weighing, etc., and achieve the effects of convenient processing, less waste, simple and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
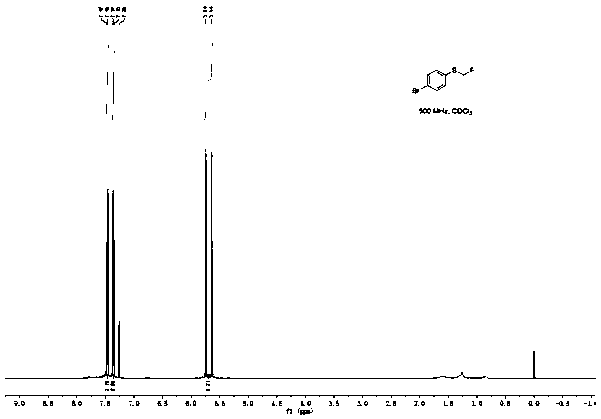
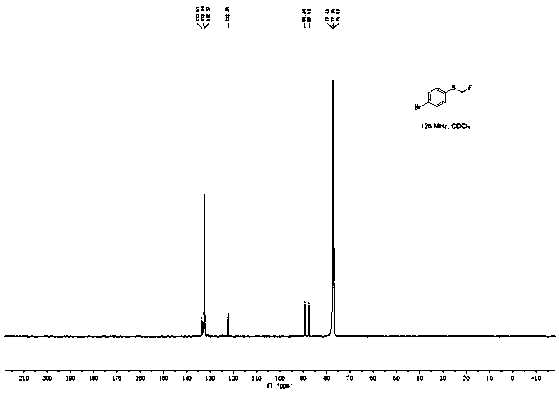
[0045]Add 100.5mg (0.5mmol) p-bromophenylboronic acid, 168.0mg (1.0mmol) Bunte salt (S-(one fluoromethyl) sodium thiosulfate), 47.5mg (0.25mmol) sulfide iodide to a 25mL pressure tube Copper, 45.0 mg (0.25 mmol) 1,10-phenanthroline, 138.0 mg (1.0 mmol) potassium carbonate, and 2 mL (50 mmol) methanol. The reaction was stirred at 90°C for 10h. After the reaction was completed, the reaction solution was diluted with 20 mL of ethyl acetate, washed three times with saturated brine, the organic layer was separated, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The crude product was separated by column chromatography (the eluent was a mixture of ethyl acetate and petroleum ether, the volume ratio of the two was 1:20) to obtain 83.6 mg of 4-fluoromethylthiobromobenzene with a yield of 76%, and NMR The characterization data are as follows:
[0046] 1 H NMR (500MHz, CDCl 3 )δ7.47(d, J=8.5Hz, 2H), 7.36(d, J=8.5Hz, 2H), 5.69(d, J=52.7H...
Embodiment 2
[0050] Add 73.5mg (0.5mmol) 4-cyanophenylboronic acid, 168.0mg (1.0mmol) Bunte salt (S-(one fluoromethyl) sodium thiosulfate), 47.5mg (0.25mmol) iodine to a 25mL pressure tube Cuprous chloride, 45.0 mg (0.25 mmol) 1,10-phenanthroline, 138.0 mg (1.0 mmol) potassium carbonate and 2 mL (50 mmol) methanol. The reaction was stirred at 90°C for 10h. After the reaction was completed, the reaction solution was diluted with 20 mL of ethyl acetate, washed three times with saturated brine, the organic layer was separated, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The crude product was separated by column chromatography (the eluent was a mixture of ethyl acetate and petroleum ether, the volume ratio of which was 1:20) to obtain 60.1 mg of 4-fluoromethylthiobenzonitrile with a yield of 72%. The NMR characterization data are as follows:
[0051] 1 H NMR (500MHz, CDCl 3 )δ7.61(d, J=8.5Hz, 2H), 7.53(d, J=8.5Hz, 2H), 5.80(d, J=52.3Hz, 2H...
Embodiment 3
[0055] Add 95.0mg (0.5mmol) 4-trifluoromethylphenylboronic acid, 168.0mg (1.0mmol) Bunte salt (S-(one fluoromethyl) sodium thiosulfate), 47.5mg (0.25mmol) to a 25mL pressure tube ) cuprous iodide, 45.0 mg (0.25 mmol) 1,10-phenanthroline, 138.0 mg (1.0 mmol) potassium carbonate and 2 mL (50 mmol) methanol. The reaction was stirred at 90°C for 10h. After the reaction was completed, the reaction solution was diluted with 20 mL of ethyl acetate, washed three times with saturated brine, the organic layer was separated, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The crude product was separated by column chromatography (the eluent was a mixture of ethyl acetate and petroleum ether, the volume ratio of the two was 1:20) to obtain 73.5 mg of 4-fluoromethylthiobenzotrifluoride, with a yield of 70%. The NMR characterization data are as follows:
[0056] 1 H NMR (500MHz, CDCl 3 )δ7.60–7.56(m,4H),5.78(d,J=52.5Hz,2H);
[0057] 13 C N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com