Detection method for epichlorohydrin and application thereof
A technology of epichlorohydrin and a detection method, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as inapplicability, and achieve the effect of simple operation and high detection accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] 1. Instruments: Agilent 7890B gas chromatograph, FID detector, Agilent 7697A headspace sampler, electronic balance
[0052] 2. Sample configuration
[0053] Accurately weigh the appropriate amount of epichlorohydrin, and dilute it with water to an epichlorohydrin solution containing 1.74 μg of epichlorohydrin per 1 mL;
[0054] Precisely pipette 5mL into a 20mL headspace bottle, seal it with a gland, and use it as a control solution;
[0055] Accurately weigh 0.5 g of propranolol hydrochloride raw material, put it in a 20 mL headspace bottle, accurately pipette 5 mL of epichlorohydrin reference solution, press the cap and shake well, and use it as the test solution.
[0056] 3. Experimental conditions
[0057] The chromatographic column is DB-624 (30m×0.32mm×1.8μm);
[0058] The carrier gas is nitrogen and the flow rate is 3mL / min;
[0059] The air flow rate is 300mL / min;
[0060] The hydrogen flow rate is 30mL / min;
[0061] Injection port 200°C;
[0062] FID detec...
Embodiment 2
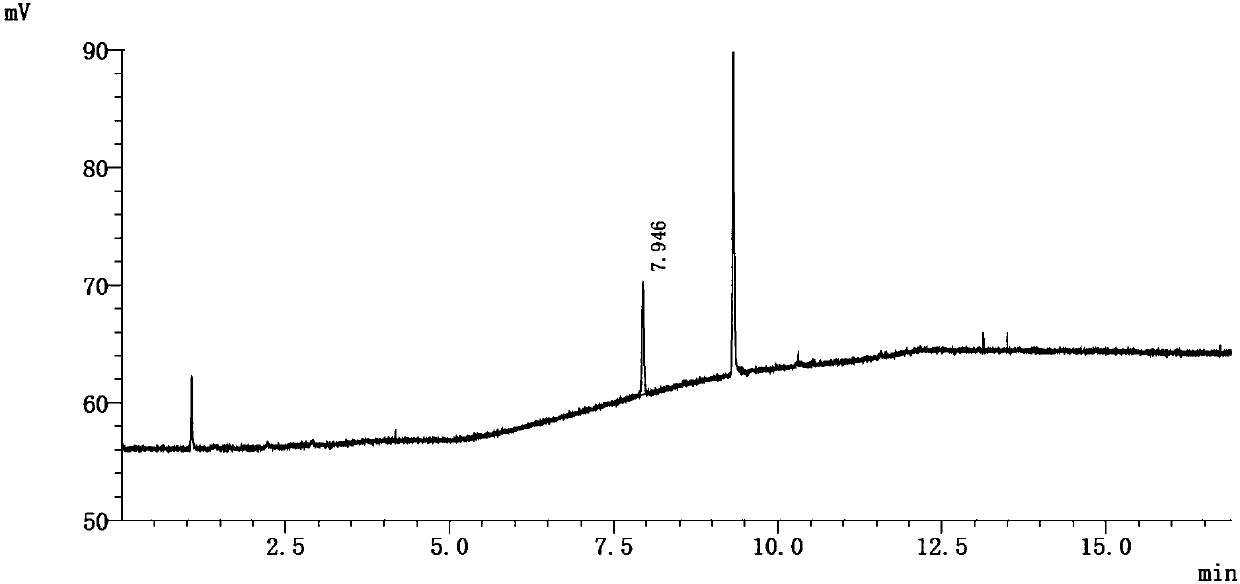
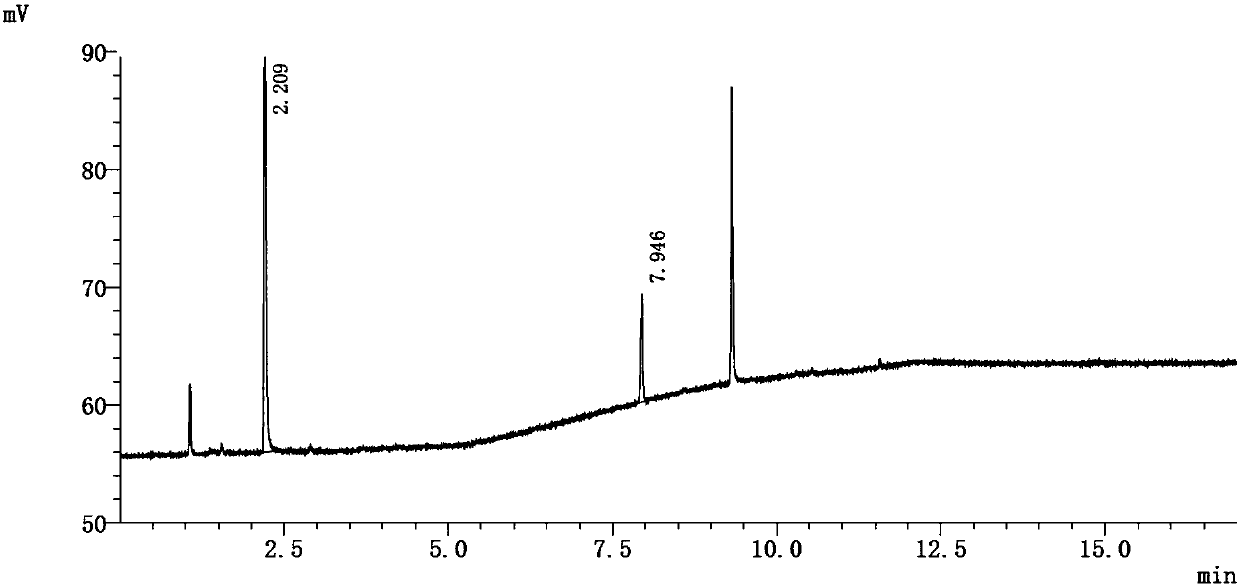
[0082] Referring to the operation of Example 1, the only difference is that the headspace equilibrium temperature is 58° C., and the equilibrium time is 10 minutes. See attached chromatogram image 3 ~ attached Figure 4 .
[0083] The chromatographic data of the contrast solution are shown in Table 3 below:
[0084] table 3
[0085]
[0086] Peak number 1 represents epichlorohydrin.
[0087] The chromatographic data of the test solution are shown in Table 4 below:
[0088] Table 4
[0089]
[0090] Peak number 1 represents epichlorohydrin.
[0091] The results show that in this method, the limit concentration of epichlorohydrin responds well, and the recovery rate of epichlorohydrin in the test solution is 89% calculated by the external standard method.
Embodiment 3
[0093] Referring to the operation of Example 1, the only difference is that the headspace equilibrium temperature is 62° C., and the equilibrium time is 10 minutes.
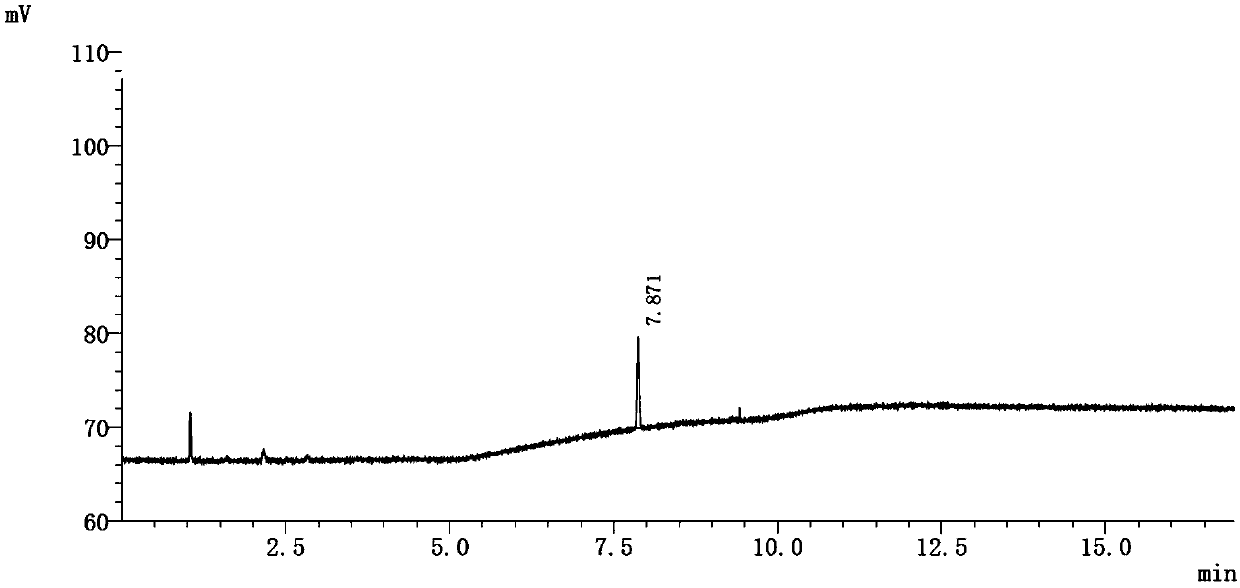
[0094] See attached chromatogram Figure 5 ~ attached Image 6 .
[0095] The chromatographic data of contrast solution are shown in table 5 below:
[0096] table 5
[0097]
[0098] Peak number 1 represents epichlorohydrin.
[0099] The chromatographic data of the test solution are shown in Table 6 below:
[0100] Table 6
[0101]
[0102] Peak number 1 represents epichlorohydrin.
[0103] The results show that in this method, the limit concentration of epichlorohydrin responds well, and the recovery rate of epichlorohydrin in the test solution is 80% calculated by the external standard method.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Column length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com