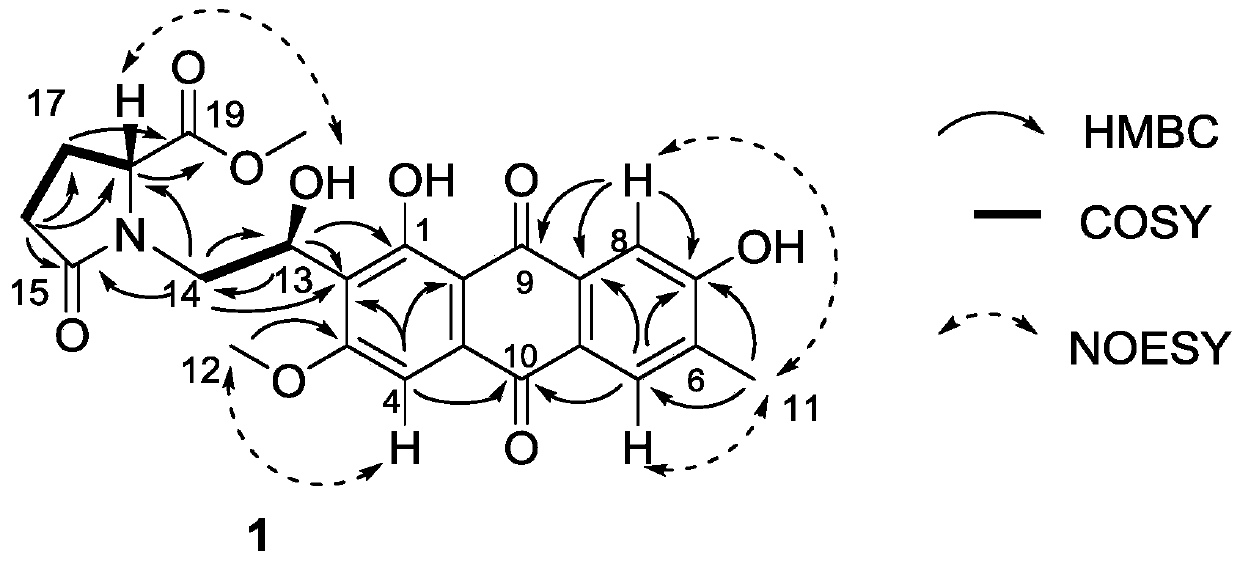
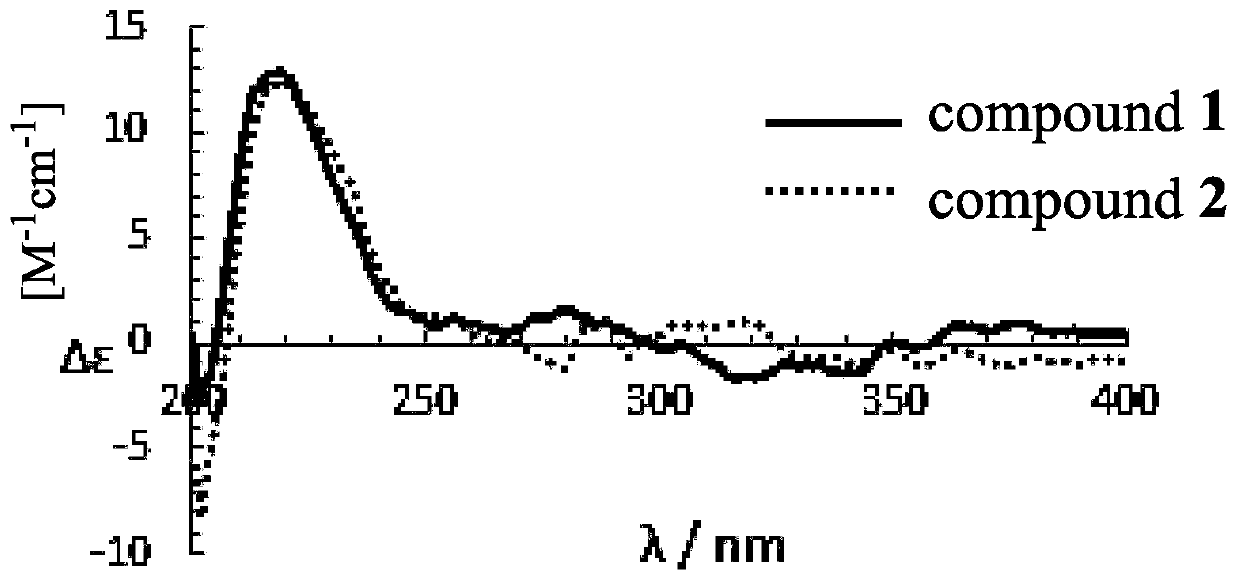
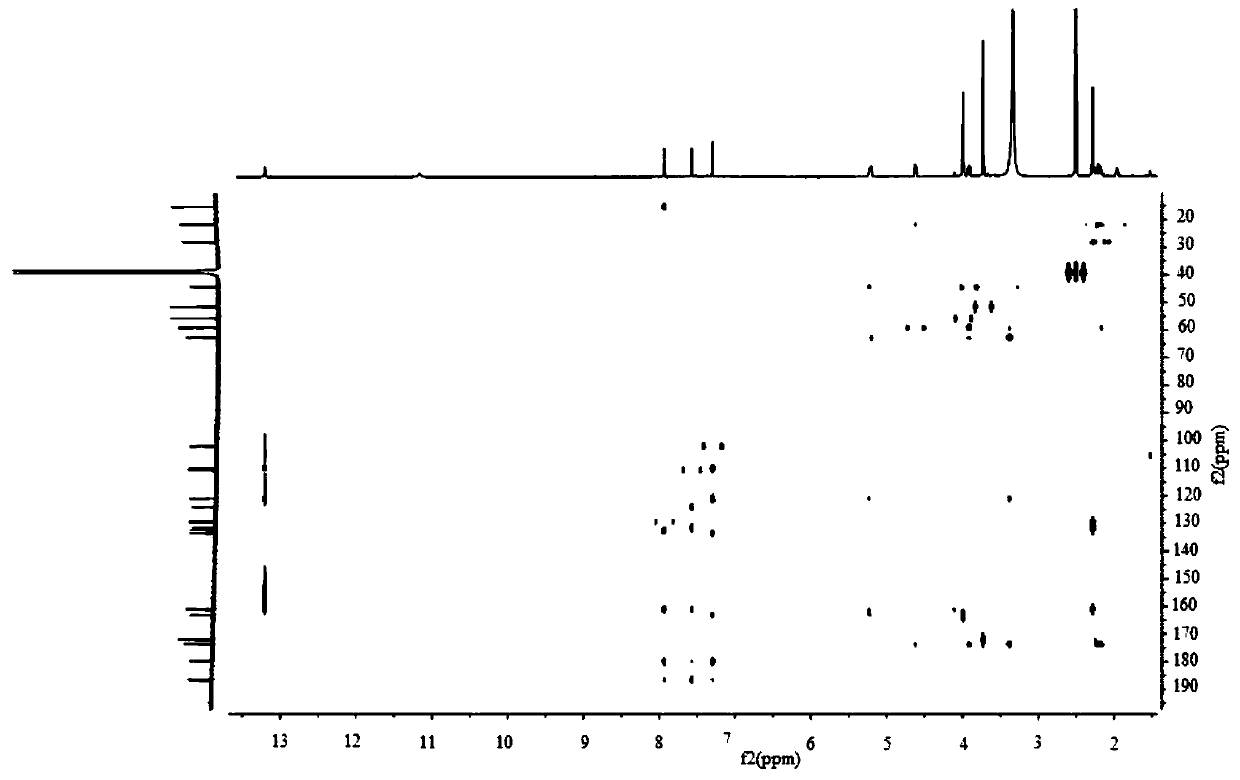
Anthraquinone compound, its preparation method and application in preparation of enzyme inhibitor
A compound and inhibitor technology, applied in the field of marine biology, can solve problems such as easy induction of drug resistance and large adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Preparation of fermentation medium: 10g of glucose, 20g of mannitol, 20g of maltose, 0.5g of corn starch, 10g of monosodium glutamate, KH 2 PO 4 0.5g, 3g of yeast extract, and 30g of sea salt were mixed, the volume was adjusted to 1L with water, and the pH was adjusted to 6.5 to obtain 1L of medium, and the medium was configured in this way. The culture medium was put into about 200 1000-mL Erlenmeyer flasks, each of about 300 mL, and autoclaved at 115° C. for 25 minutes.
[0033](2) Preparation of fermentation broth: The fungus A.tenuissma JX156349 was grown in a plate medium suitable for fungi, and after the fungi grew spores, the fungi were moved from the plate to a triangular flask filled with water with a bamboo stick, and the The fungus was inoculated into the fermentation medium by a liquid gun (300 mL of fermentation medium was contained in a 1L conical flask), and the fermentation broth was collected after standing at room temperature (26° C.) for 30 days...
Embodiment 2
[0050] Phosphokinase and IDO1 Enzyme Inhibitory Activity Test of the Compounds of Example 2
[0051] (1) Phosphokinase inhibitory activity assay: Human phosphokinases PTP1B, SHP1, SHP2, MEG2 or TCPTP were cloned into Escherichia coli and purified. Enzyme inhibitory activity was assayed in 96-well plates using p-nitrophenyl phosphate (pNPP) as substrate with 100 μL of reaction mixture per well. Human recombinant PTP1B, SHP1, SHP2, MEG2orTCPTP (0.05 μg) was added to 50 μL of reaction buffer (pH 6.5) containing 50 mM HEPES, 100 mM NaCl, 1 mM EDTA, and 1 mM dithiothreitol (DTT), and each 96-well plate was separated Add samples of compounds to be tested (compounds Anthrininone A, Anthrininone B, Anthrininone C and 6-O-methylalaternin). Na 3 VO 4 As a positive control, DMSO was used as a negative control to evaluate this high-throughput screening system. After a 15 min pre-incubation at room temperature, 50 μL of buffer containing 50 mM pNPP was added and the incubation was cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com