Conjugate of minoxidil and peptide
A technology of compound and composition, applied in the field of combination of minoxidil and peptide, to achieve excellent water stability, excellent skin penetration rate, and the effect of improving hair loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Synthesis and solubility evaluation of the compound of the present invention
[0071] Synthesis of peptides
[0072] Synthesis of the peptide of SEQ ID No: 3
[0073] 700 mg of chlorotrityl chloride resin (CTL resin; Nova biochem [0064] classification No. 01-64-0021) was placed in the reactor, and 10 ml of dichloromethane (MC) was added to the reactor, and then Stir for 3 minutes. The solution was removed and 10 ml of dimethylformamide (DMF) was added to the reactor. After stirring for 3 minutes, the solvent was removed again. 10 ml of dichloromethane (DCM) was added to the reactor, and then 200 mmol of Fmoc-Cys(trt)-OH (Bachem, Swiss) and 400 mmol of diisopropylethylamine (DIEA) were added and stirred to fully dissolve , and reacted with stirring for 1 hour. After the reaction, washing was performed, and methanol and DIEA (2:1) were dissolved in DCM, reacted for 10 minutes, and washed with excess DCM / DMF (1:1). The solution was removed and 10 ml of dimet...
Embodiment 2
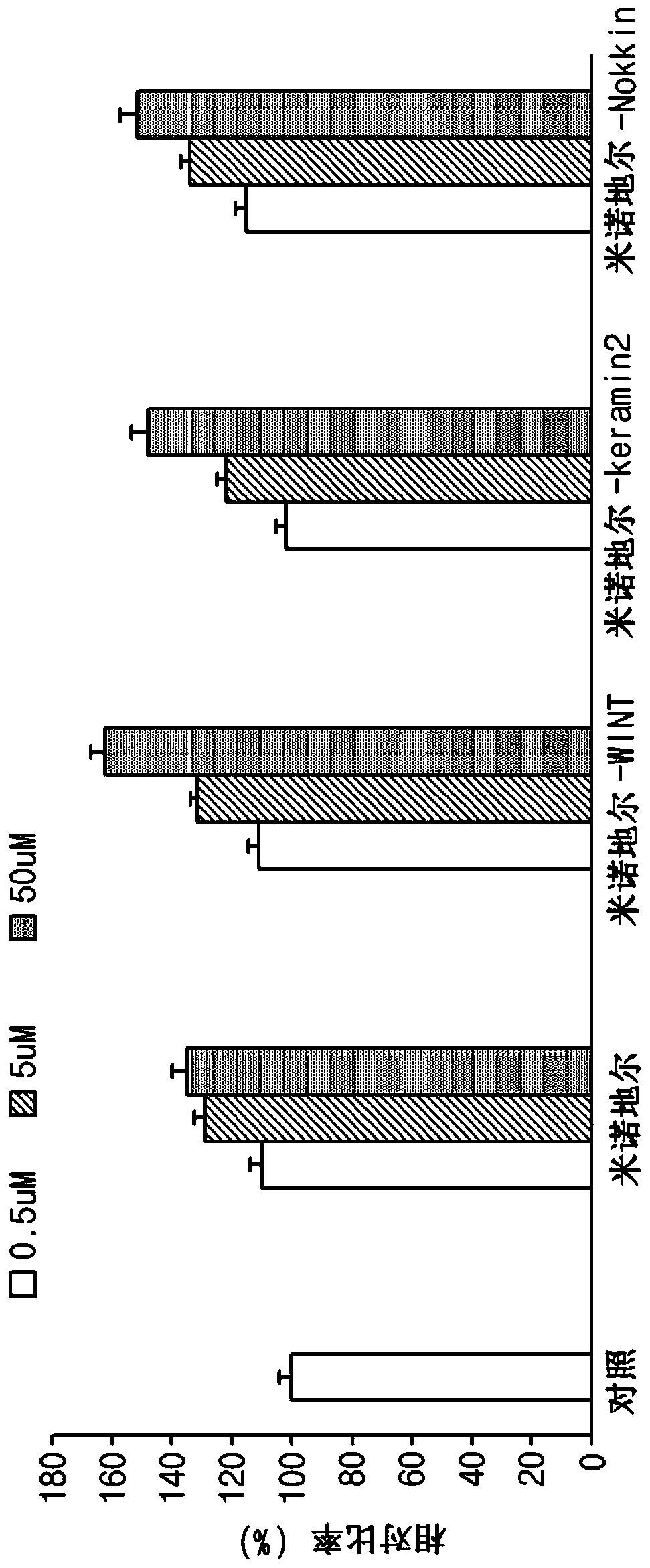
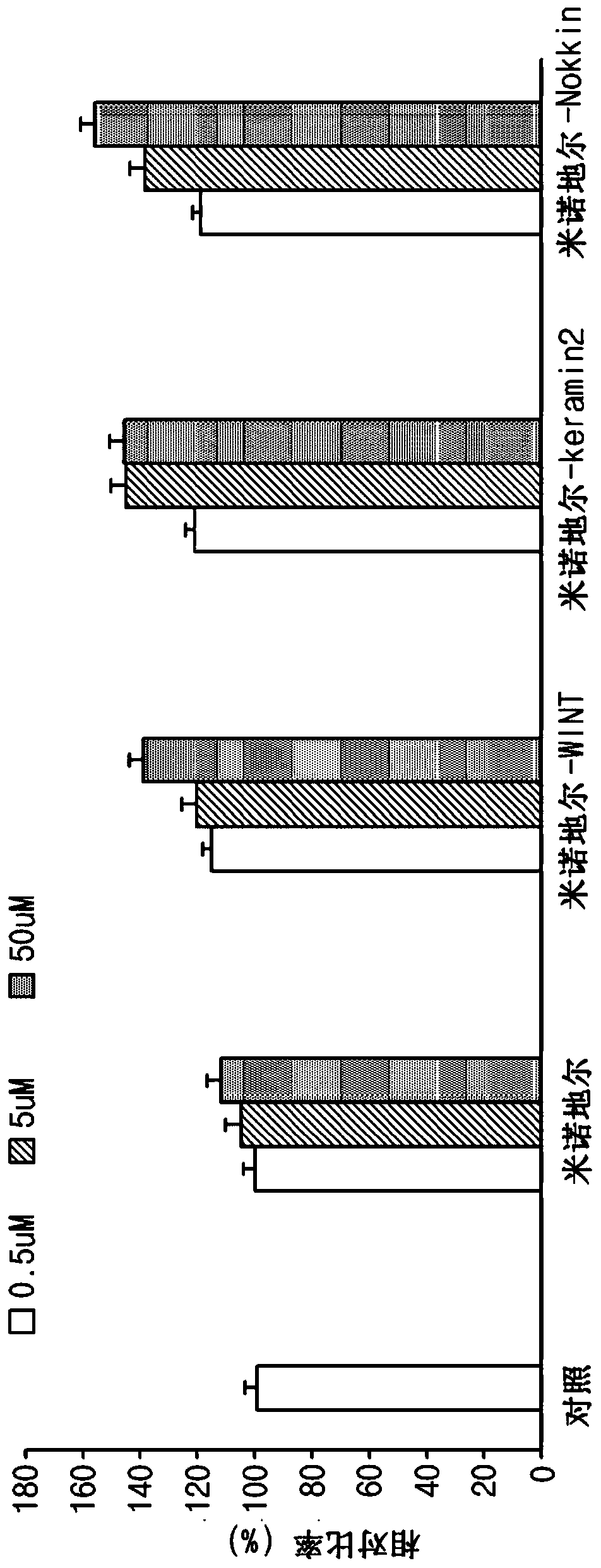
[0093] Evaluation of the degree of cell proliferation when treated with the compound of the present invention
[0094] Evaluation of cell proliferation of human umbilical vein endothelial cells (HUVEC)
[0095] In order to confirm the function of the compound of the present invention synthesized in Example 1, HUVEC were treated with the compound of the present invention to determine the degree of proliferation. Place 3000 HUVECs in each well of a 96-well plate and store in CO 2Incubate for 24 hours in the incubator. After 24 hours, the medium was replaced with serum-free DMEM medium, and the three compounds of the present invention synthesized in Example 1 and minoxidil were added to the cells at a concentration of 0.5uM, 5uM and 50uM respectively, Incubate for 72 hours. After the culture was completed, the culture supernatant was removed, and the cells were fixed with ethanol and washed three times with PBS (phosphate buffered saline). After removing the washing sol...
Embodiment 3
[0100] Evaluation of the Effects of the Compounds of the Present Invention on the Expression of VEGF and TGFβ1
[0101] Since VEGF plays a role in angiogenesis and dilation and TGFβ1 affects alopecia, the expression levels of VEGF and TGFβ1 upon treatment with the compounds of the present invention were confirmed.
[0102] Evaluation of the amount of mRNA (transcription level)
[0103] VEGF is expressed by HUVECs, whereas TGFβ1 is expressed by hair dermal papilla cells. Two kinds of cells were divided into 1×10 5 The ratio of cells / well was placed in each plate of a 6-well plate. in CO 2 After culturing in the incubator for 24 hours, the medium was replaced with serum-free DMEM medium. The three compounds of the present invention and minoxidil were added to the cells at concentrations of 5uM and 50uM, respectively, and incubated for 24 hours. After the cultured cells were harvested, RNA was extracted using an RNA extraction kit, and RT-PCR was performed to confirm t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com