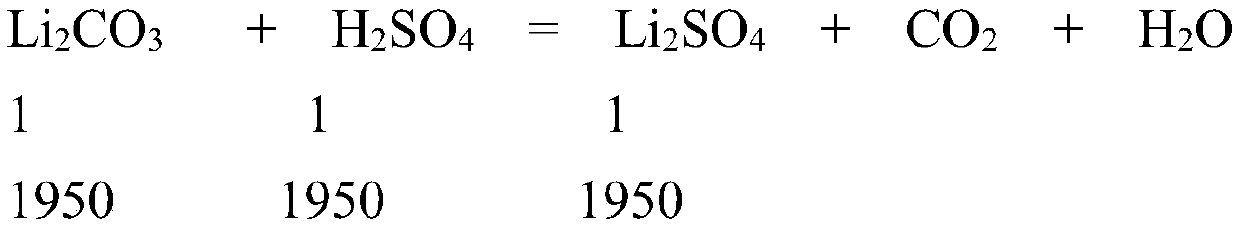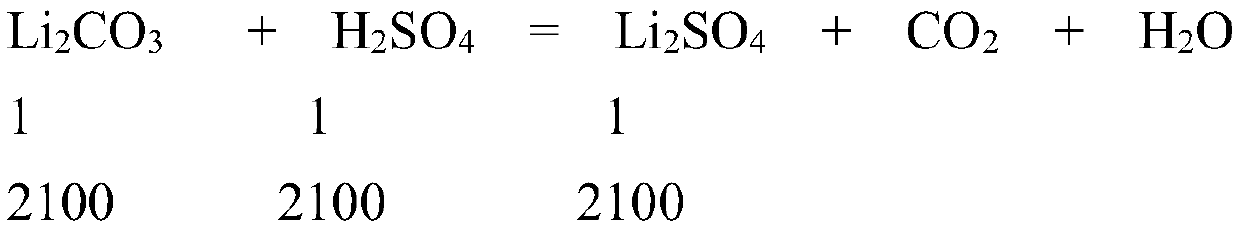Method for preparing lithium hydroxide by industrial-grade lithium carbonate solid
A technology of lithium hydroxide and lithium carbonate, applied in electrolysis process, electrolysis components, etc., can solve the problem of not being able to obtain battery-grade lithium hydroxide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Batching concentration 1.3mol / L; Batching volume 1.5m 3 ; then contains Li 2 SO 4 The amount is 1.3mol / L×1.5m 3 =1950mol;
[0055] Then the amount of required industrial grade lithium carbonate is:
[0056]
[0057] Lithium carbonate molar mass is 74g / mol; The molar mass of sulfuric acid is 98g / mol; The raw material uses technical grade Lithium carbonate main content in 99%; Sulfuric acid concentration used is 98%, and excess coefficient is 1.05; Then required raw material quality is:
[0058] (1) Li 2 CO 3 : 1950×74÷99%=145758g≈145.8kg;
[0059] (2)H 2 SO 4 : 1950×98÷98%×1.05=204750g≈204.8kg;
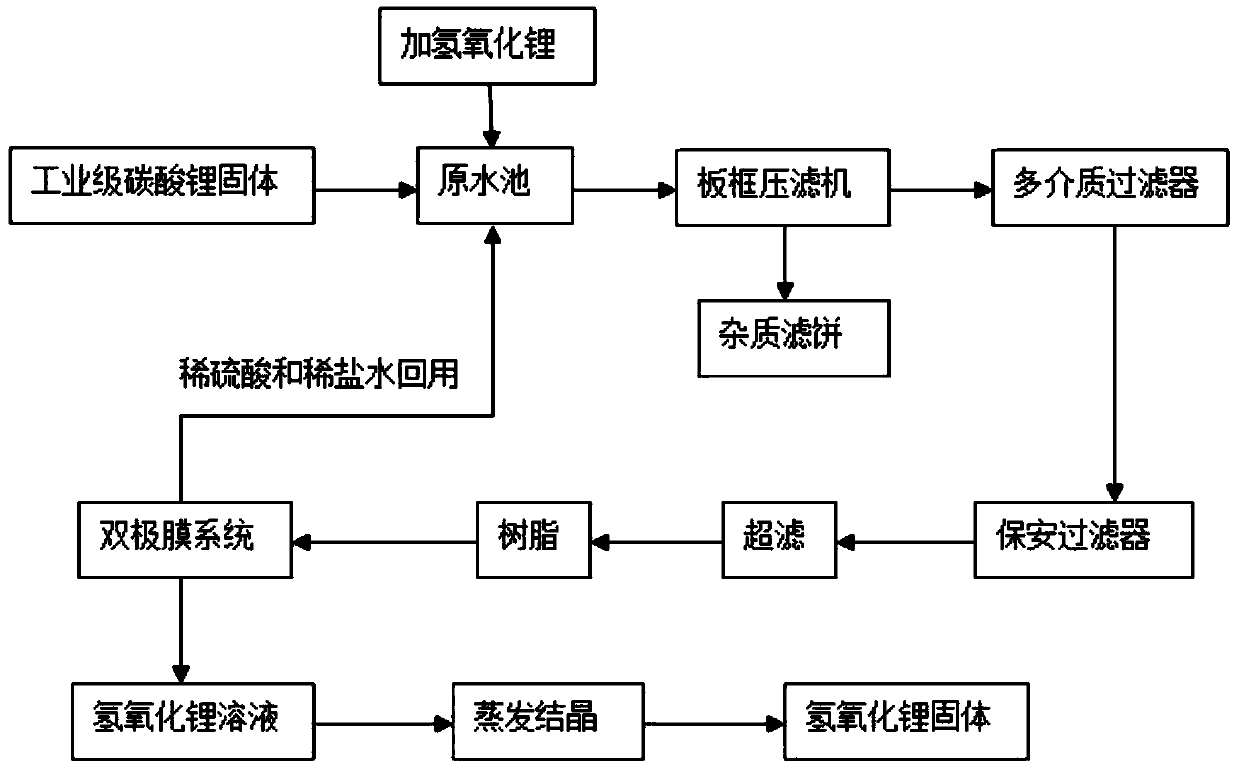
[0060] Industrial grade Lithium carbonate solid is mixed with sulfuric acid to carry out metathesis reaction, obtain metathesis reaction product, metathesis reaction product is adjusted pH value to 7.5 through lithium hydroxide successively, plate and frame filtration (the pressure of plate and frame filtration is 1.6 MPa, filter frame aperture is 3 microns), multi-...
Embodiment 2
[0063] Batching concentration 1.4mol / L; Batching volume 1.5m 3 ; then contains Li 2 SO 4 The amount is 1.4mol / L×1.5m 3 =2100mol;
[0064] Then the amount of required industrial grade lithium carbonate is:
[0065]
[0066] Lithium carbonate molar mass is 74g / mol; The molar mass of sulfuric acid is 98g / mol; The raw material uses technical grade Lithium carbonate main content in 99%; Sulfuric acid concentration used is 98%, and excess coefficient is 1.05; Then required raw material quality is:
[0067] (1) Li 2 CO 3 : 2100×74÷99%=156970g≈156.9kg;
[0068] (2)H 2 SO 4 : 2100×98÷98%×1.05=220500g≈220.5kg;
[0069] The metathesis reaction product is successively adjusted to pH 10 by lithium hydroxide, plate and frame filtration (the pressure of plate and frame filtration is 3 MPa, and the aperture of the filter frame is 8 microns), multi-media filtration (activated carbon, quartz sand and porous ceramics, etc.), After ultrafiltration (pore size is 0.05 micron) and chela...
Embodiment 3
[0072] Batching concentration 1.5mol / L; Batching volume 1.5m 3 ; then contains Li 2 SO 4 The amount is 1.3mol / L×1.5m 3 =2250mol;
[0073] Then the amount of required industrial grade lithium carbonate is:
[0074]
[0075] Lithium carbonate molar mass is 74g / mol; The molar mass of sulfuric acid is 98g / mol; The raw material uses technical grade Lithium carbonate main content in 99%; Sulfuric acid concentration used is 98%, and excess coefficient is 1.05; Then required raw material quality is:
[0076] (1) Li 2 CO 3 : 2250×74÷99%=168181g≈168.2kg;
[0077] (2)H 2 SO 4 : 2250×98÷98%×1.05=236250g≈236.3kg;
[0078] The metathesis reaction product is successively adjusted to a pH value of 13 by lithium hydroxide, plate and frame filtration (the pressure of the plate and frame filtration is 2 MPa, and the pore size of the filter frame is 10 microns), multi-media filtration (activated carbon, quartz sand, porous ceramics, etc.) , after ultrafiltration (pore size is 0.02 mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com