A deuterium labeled d 3 -synthetic method of albuterol
A technology for salbutamol and a synthesis method, applied in the fields of food safety and standard product synthesis, can solve problems such as being unsuitable for synthesis, and achieve the effects of easy purification, simple operation and simple processing method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
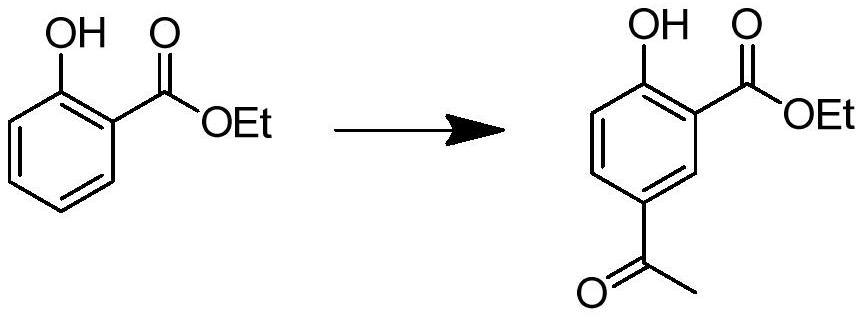
[0041](1) Preparation of ethyl 2-hydroxy-5-acetylbenzoate
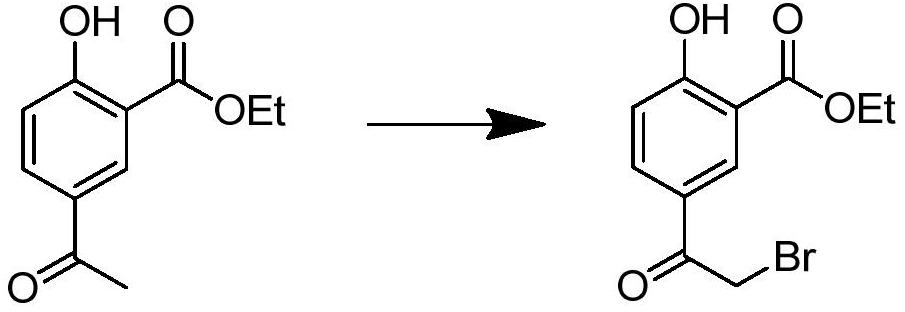
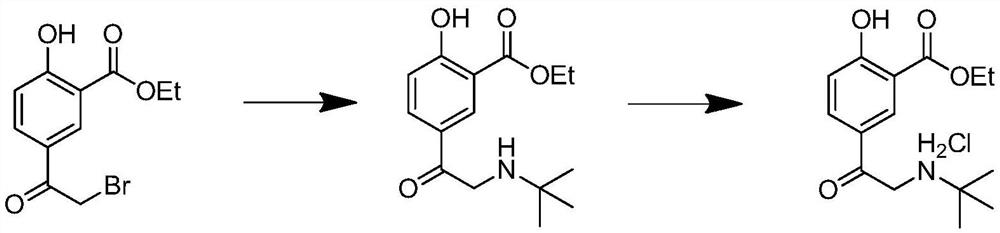
[0042] Add 30 g (0.181 mol) of ethyl salicylate and 300 ml of dichloromethane into a 500 ml three-necked flask, cool to 0° C., add 18.4 g (0.231 mol) of acetyl chloride therein, and stir to obtain a mixed solution. Weigh anhydrous AlCl 3 60.4g (0.457mol), in order to avoid violent exotherm, add to the mixed solution in stages, add about 6g of aluminum trichloride each time, add about 10 times, each interval is about 3 minutes, and naturally rise to room temperature after adding React for 6 hours, slowly pour the reacted reaction solution into 300g ice cubes, stir and separate layers, take the organic phase and wash it twice with water, then wash it once with saturated sodium bicarbonate solution, wash it once with saturated saline, wash it once with Dry the organic layer with anhydrous sodium sulfate, remove the solvent under reduced pressure, recrystallize with n-hexane, filter with suction, and dry to obtain 33.2 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com