Tumor vaccine and preparation method thereof
A tumor vaccine, tumor technology, applied in the field of tumor vaccine and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The preparation of this application tumor vaccine
[0071] 1. Experimental Materials and Reagents
[0072] The C57BL / 6 mice used in the experiment were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., aged 6-8 weeks, weighing about 18g.
[0073] Fetal calf serum (FCS): purchased from PAN company;
[0074] RPMI-1640 medium: purchased from PAN company;
[0075] GM-CSF and IL-4 were purchased from Peprotech, USA;
[0076] 2. Experimental steps
[0077] (1) Isolating mouse femur bone marrow hematopoietic precursor cells;
[0078] (2) The isolated mouse bone marrow hematopoietic precursor cells were mixed with 2×10 6 / mL density cultured in RPMI-1640 medium supplemented with 50ng / mL GM-CSF, 50ng / mL IL-4, 10% fetal calf serum (FCS), temperature 37°C, CO 2 The concentration is 5v / v%, induced for 7 days, the cell pellet is collected, the sample is resuspended with normal saline, and the smear is observed under a microscope. The results are as...
Embodiment 2
[0083] The preparation of this application tumor vaccine
[0084] 1. Experimental materials and reagents
[0085] The C57BL / 6 mice used in the experiment were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., aged 6-8 weeks, weighing about 18g.
[0086] Fetal calf serum (FCS): purchased from PAN company;
[0087] RPMI-1640 medium: purchased from PAN company;
[0088] GM-CSF and IL-4 were purchased from Peprotech, USA;
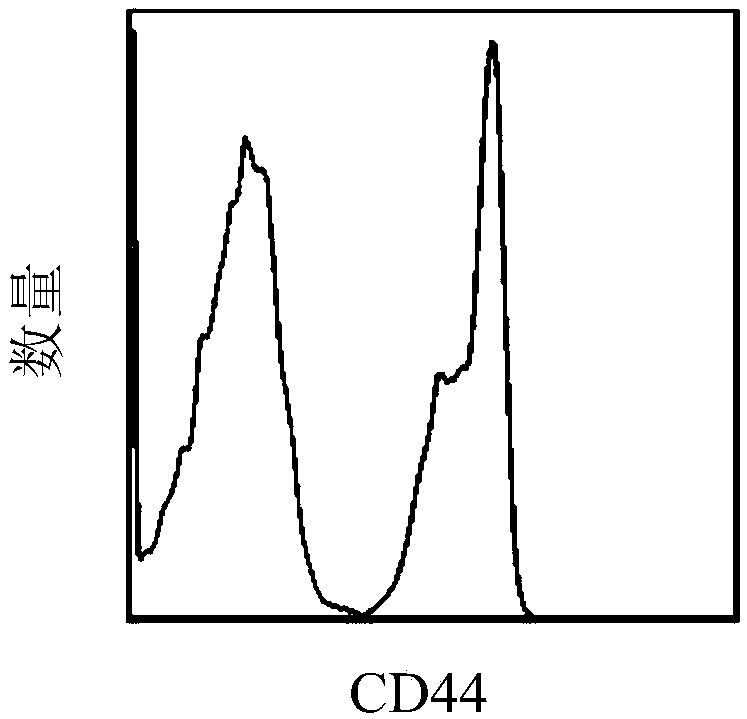
[0089] The anti-mouse CD44 antibody was purchased from Biolegend, USA, the catalog number is 156002.
[0090] 2. Experimental steps
[0091] (1) Isolating mouse femur bone marrow hematopoietic precursor cells;
[0092] (2) The isolated mouse bone marrow hematopoietic precursor cells were mixed with 2×10 6 / mL density cultured in RPMI-1640 medium supplemented with 50ng / mL GM-CSF, 50ng / mL IL-4, 10% FCS, temperature 37°C, CO 2 The concentration is 5v / v%, induced for 7 days, the cell pellet is collected, the sample is resuspended...
Embodiment 3
[0097] The tumor vaccine of the present application (that is, the dendritic cells (CD44) knocking out the CD44 gene - / - DC)) and conventional dendritic cell tumor vaccine (that is, mature dendritic cells (CD44 + / + DC)) comparison of the ability to activate the immune system of the recipient.
[0098] 1. Experimental materials and reagents
[0099] The C57BL / 6 mice used in the experiment were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., aged 6-8 weeks, weighing about 18g.
[0100] Cytokines in serum were detected using Biolegend Cytokine Detection Kit (Cat. No. 740005).
[0101] anti-mouse H 2 K b ,Ia b , CD40, CD80, CD86, and CD44 fluorescent antibodies were purchased from Biolegend Company in the United States, and the article numbers were H 2 K b (114605), Ia b (116410), CD40 (124607), CD80 (104705), CD86 (105005), CD44 (156002).
[0102] The antibody used to label mouse peripheral blood CD8 cells was PE-labeled anti-CD8 antibody (a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com