Application of KMT1A inhibitor in preparation of anticancer drug for bladder cancers
An inhibitor and bladder cancer technology, applied in the field of tumor molecular biology and chemical drugs, can solve the problem of decreased STAT3 protein phosphorylation level, increased GATA3 gene transcription level, bladder cancer stem cell spheroid formation ability and tumor formation ability weakened, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1, cell proliferation experiment
[0032] (1) Bladder cancer cell lines BIU87, T24 were routinely cultured in RPMI 1640 medium with 10% fetal bovine serum.
[0033] (2) Digest BIU87, T24 cells, wash once with 5ml PBS, and count accurately.
[0034] (3) Plate 3000 cells / 100 μl / well in a 96-well plate and culture overnight.
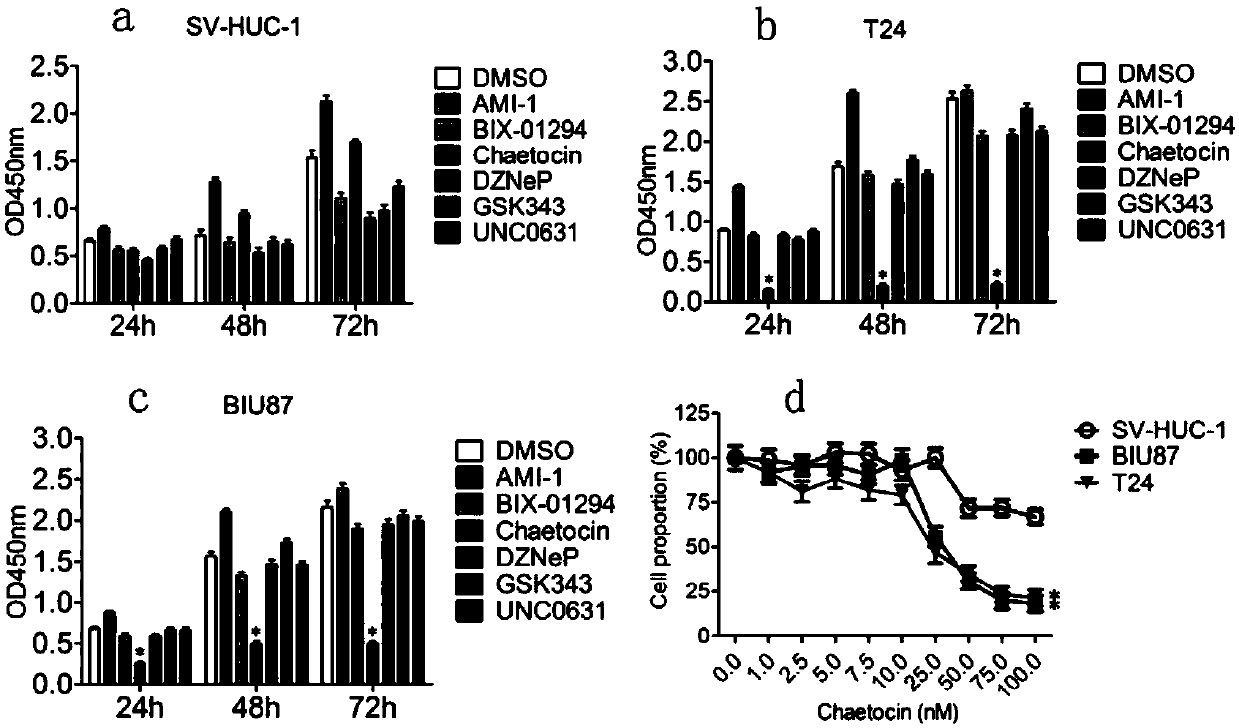
[0035] (4) The next day, after the cells adhered to the wall, replace the culture medium with 5% DMSO, 150 μM AMI-1, 2.5 μM BIX-01294, 50 nM Chaetocin, 2.5 μM DZNeP, 10 μM GSK343 and 2.5 μM UNC0631, and culture for 24 h, 48 h , 72h.
[0036] (5) At each time point, remove the original medium, add 100 μl of serum-free 1640 medium containing CCK8, incubate at 37°C for 2 hours, and measure the absorbance at 450nm (OD 450nm).
[0037] like figure 1 As shown, Chaetocin, a preferred embodiment of the KMT1A inhibitor of the present invention, can specifically inhibit the cell proliferation of bladder cancer cells.
Embodiment 2
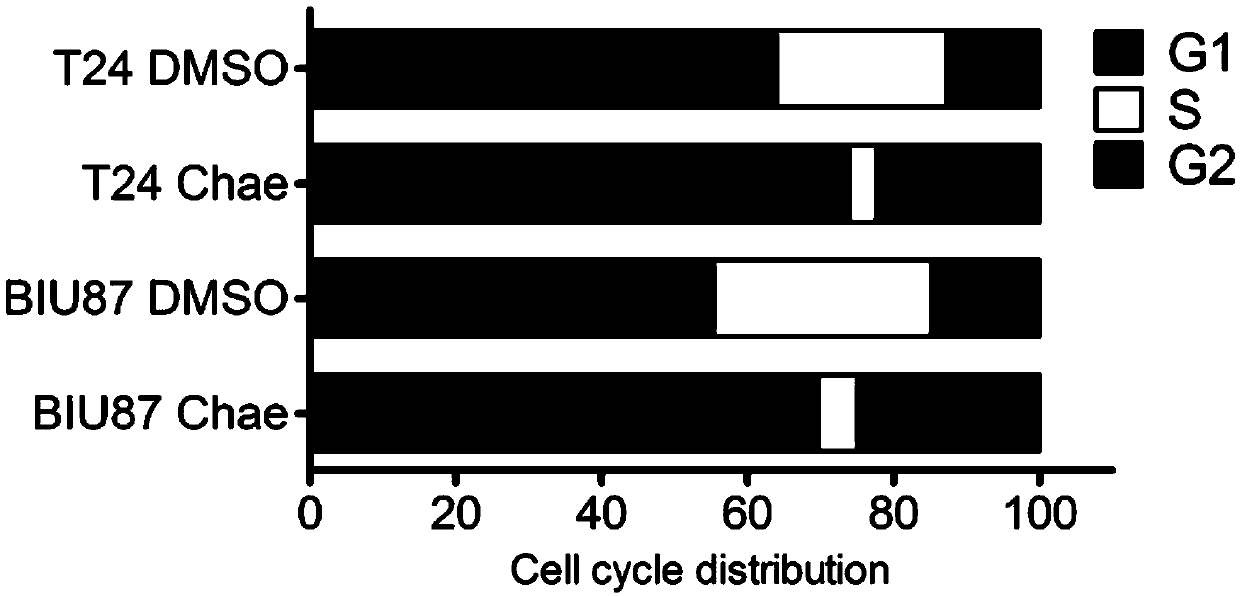
[0038] Embodiment two, cell cycle experiment
[0039] (1) The day before, BIU87 and T24 cells were mixed with 4×10 cells per well 5 Cells were seeded in a 6-well plate, and the serum-free medium was replaced in the morning after attachment the next day to synchronize the cell cycle.
[0040](2) After 24 hours, add fresh medium containing 2 μl DMSO to 3 wells, add fresh medium containing 50 nM Chae inhibitor to the other 3 wells, and culture for 24 hours and 48 hours.
[0041] (3) After 24h and 48h, collect the cells by trypsinization, wash twice with PBS, discard the supernatant, add 1ml of 70% pre-cooled ethanol, pipette evenly, and fix at -20°C overnight (>12h).
[0042] (4) Before flow detection, wash with PBS to remove ethanol, centrifuge at 3000rpm for 4min, and wash twice. Resuspend the cells with 0.5ml PBS, add RNaseA to a final concentration of 50μg / ml, and incubate at 37°C for 30min. Add 25 μl PI and mix well, incubate at 37°C for 10 minutes, and measure the period...
Embodiment 3
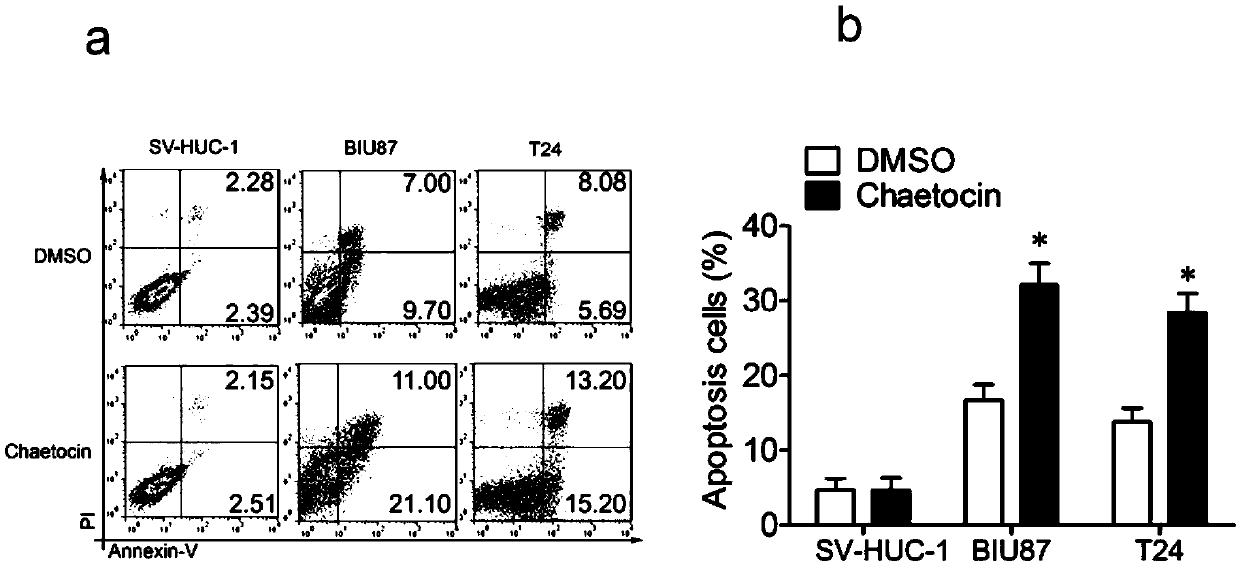
[0044] Embodiment three, cell apoptosis experiment
[0045] (1) The day before, BIU87 and T24 cells were mixed with 4×10 cells per well 5 The cells were seeded in a 6-well plate, and after adherence the next day, fresh medium containing 2 μl DMSO was added to 3 wells, and fresh medium containing 50 nM Chaetocin inhibitor was added to the other 3 wells, and cultured for 24 h and 48 h.
[0046] (2) After 24h and 48h, suck out the cell culture medium into a suitable centrifuge tube, wash the adherent cells once with PBS, and add an appropriate amount of trypsin cell digestion solution to digest the cells. Incubate at room temperature until the adherent cells can be blown down by gentle pipetting, then aspirate the trypsin cell digestion solution. Overdigestion with trypsin should be avoided.
[0047] (3) Add the cell culture medium collected in step (2), mix slightly, transfer to a centrifuge tube, centrifuge at 1000 g for 5 min, discard the supernatant, collect the cells, gent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com