Methods of priming a sus' immune system
An immune system and animal technology, applied in the field of activating the immune system of pigs, can solve the problems of time-consuming, defective, and poor convenience in separating or extracting cell wall nuclear components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0060]1. A method for activating the immune system of an animal of the genus Suis, the method comprising administering an effective amount of whole cell lysate of mycobacteria to the animal of the genus Suis within an effective period after birth of the animal of the genus Suis.
[0061] 2. The method of clause 1, wherein the mycobacterial whole cell lysate is prepared from Mycobacterium smegmatis.
[0062] 3. The method of clause 1 or clause 2, wherein the mycobacterial whole cell lysate has not been purified.
[0063] 4. The method of any one of clauses 1 to 3, wherein the mycobacterial whole cell lysate is not fractionated.
[0064] 5. The method of any one of clauses 1 to 4, wherein the mycobacterial whole cell lysate is lipid-free.
[0065] 6. The method of any one of clauses 1 to 5, wherein the mycobacterial whole cell lysate is deproteinized.
[0066] 7. The method of any one of clauses 1 to 6, wherein the administering is selected from the group consisting of oral, i...
Embodiment 1
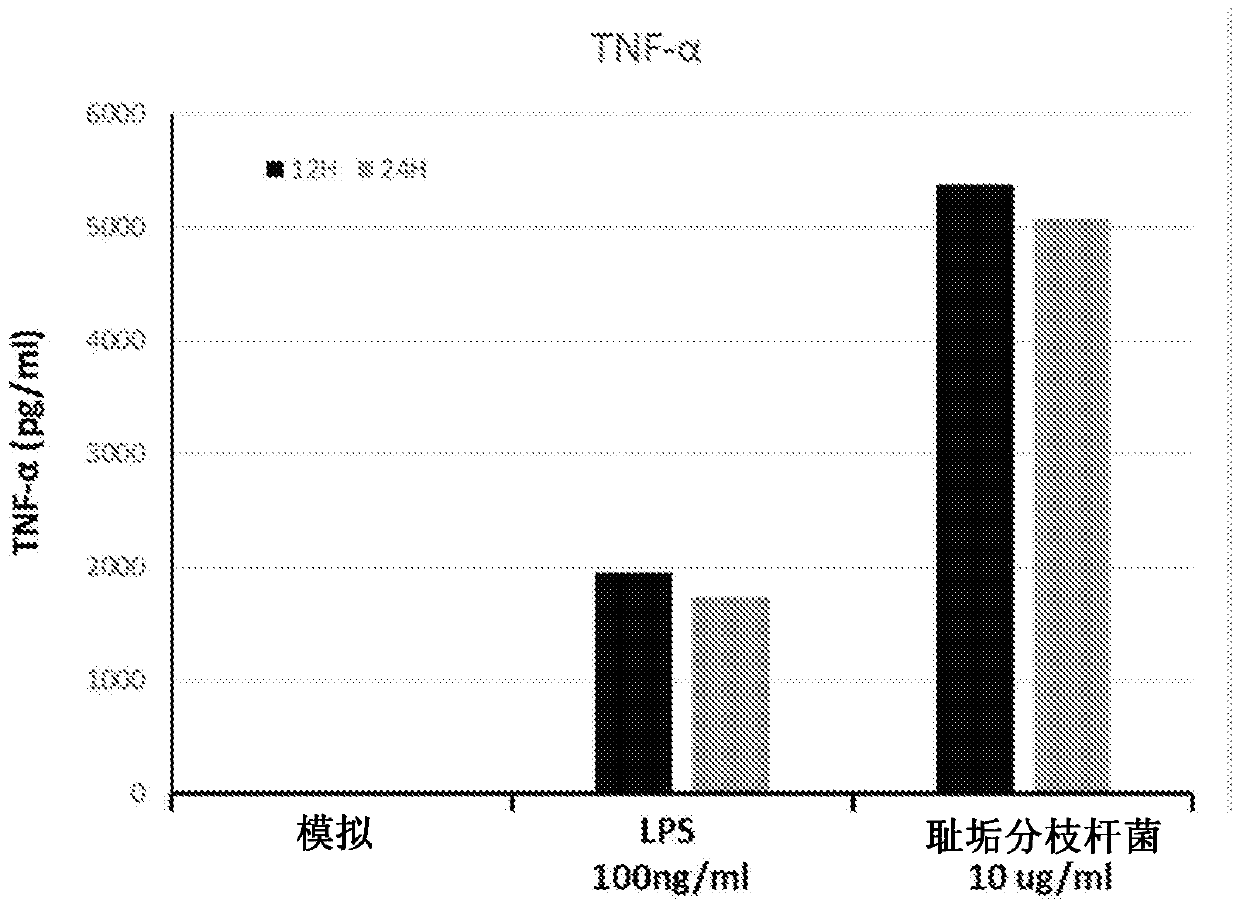
[0189] Significant production of TNF-α by porcine AMΦ stimulated with M. smegmatis WCL
[0190] The ability of ZMAC cells to produce TNF-α in response to LPS has been shown in previous studies. To test the immunostimulatory activity of M. smegmatis WCL, ZMAC cells were exposed to M. smegmatis WCL grown in 7H9 broth optimized for mycobacterial cultures. Cells were initially stimulated with a high concentration of 10 ug / mL M. smegmatis for 12 and 24 hours. Such as figure 1 As shown, the results demonstrate a lower production of TNF-α when porcine AMΦZMAC is exposed to the bacterial product lipopolysaccharide (LPS), which is a potent stimulator of TNF-α production, when porcine alveolar enlargement Burst production of tumor necrosis factor (TNF)-α by phagocytes (AMΦ) ZMAC exposed to M. smegmatis WCL.
Embodiment 2
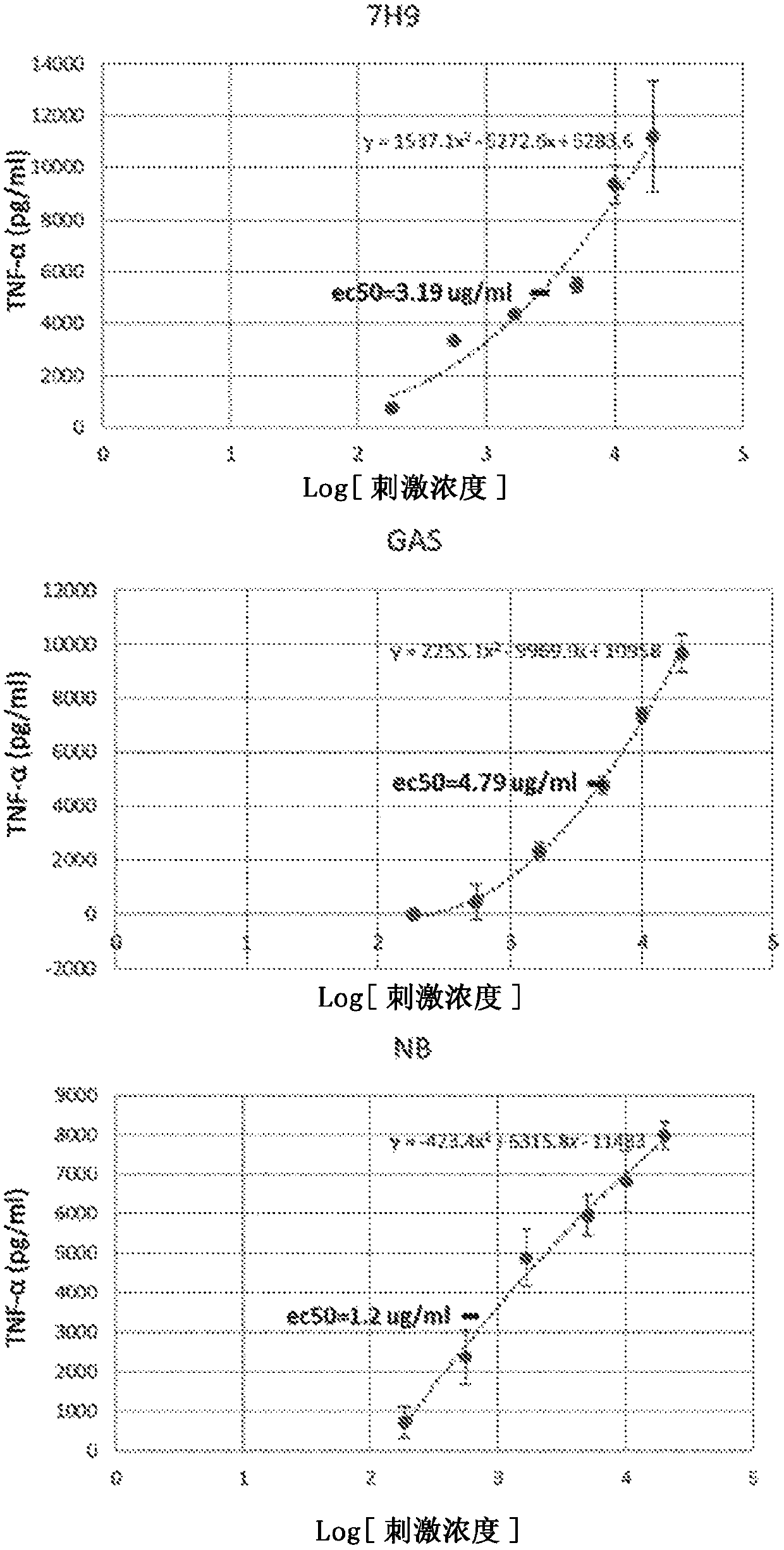
[0192] Most TNF-α production by AMΦ in response to M. smegmatis WCL occurs within the first 6 hours after stimulation pregnancy
[0193] While data from previous experiments showed the production of significant amounts of TNF-[alpha] 12 hours post-stimulation, there appeared to be no further production of this cytokine during the 12-24 hour period. This finding led the inventors of the present disclosure to question whether the response of TNF-α to M. smegmatis WCL resembles the similar expression kinetics that have been observed for the response of this cytokine to LPS stimulation, which typically occurs within 4 days after stimulation. - peak within 6 hours. Therefore, a time analysis was set up to establish the kinetics of TNF-α production in response to stimulation of M. smegmatis WCL. In this experiment, the inventors of the present disclosure also included two other WCLs prepared from the same M. smegmatis but cultured in different broth types. Such as figure 2 A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com