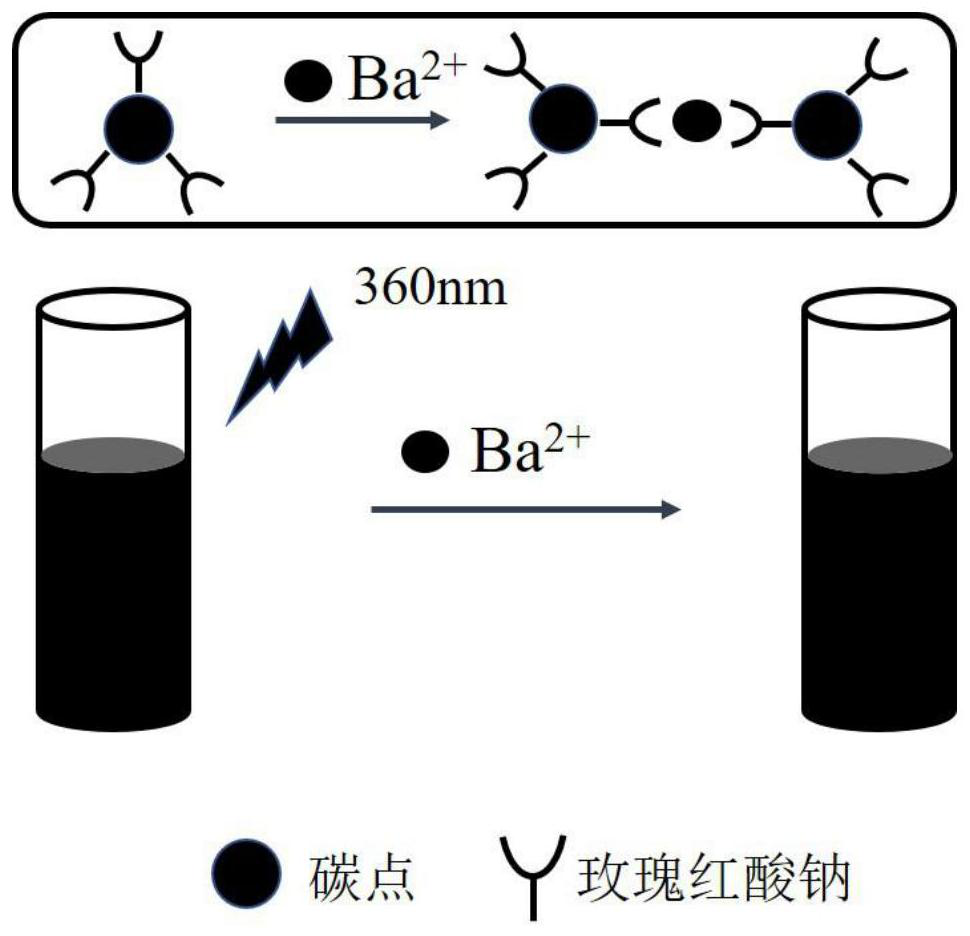
A kind of preparation method of the fluorescent test paper of rapid detection barium ion
A barium ion and fluorescence technology, which is applied in the field of analytical chemical testing, can solve the problems of substandard detection sensitivity, expensive instruments, and interference from other ions, and achieve the effects of wide discoloration range and high sensitivity that are conducive to promotion and popularization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 The preparation method of the fluorescent test paper that detects barium ion rapidly
[0021] (1) Dissolve 1.0-5.0g of citric acid and 2-10mL of ethylenediamine in 20mL of ultrapure water, then transfer to a 30mL polytetrafluoroethylene reactor, and react at a temperature below 200°C for 4-8h to obtain blue light carbon dots. The obtained blue-light carbon dots were dialyzed for 24-48 hours with a dialysis bag with a cut-off molecular weight of 500;
[0022] (2) Disperse 10 mL of dialyzed blue light carbon dots in a 10.0-30.0 μmol / L sodium rhodinate solution, and stir for 1-3 hours to obtain a fluorescent probe solution.
[0023] (3) Wash the ink cartridge of the inkjet printer with ultrapure water, dry it to obtain a blank ink cartridge, inject the fluorescent probe solution into the blank ink cartridge with a syringe, and print a 7×2cm ink cartridge on ordinary filter paper. 2 Rectangle pattern, repeat printing 15-25 times, leave it for 8-12 minutes natu...
Embodiment 2
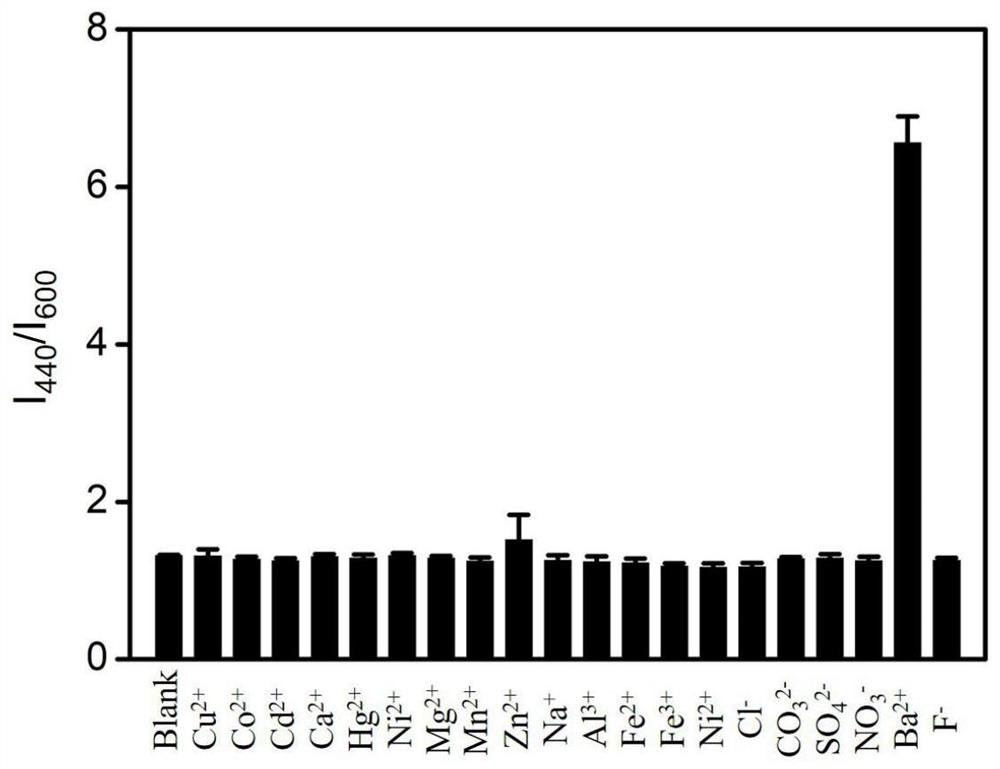
[0024] Embodiment 2 Barium ion adds the influence of different amounts on color development
[0025] Take the concentration as 1.0×10 -5 mol / L barium chloride standard solution 0.00mL, 0.05mL, 0.10mL, 0.20mL, 0.50mL, 1.00mL were put into six 10mL glass test tubes respectively, and deionized water 1.00mL, 0.95mL, 0.90mL were added respectively. mL, 0.80mL, 0.50mL, 0.00mL. Insert the fluorescent test paper prepared in Example 1 into the test tube to wet the test paper, (generally, there will be a color reaction within 10 minutes after wetting). Use a hand-held ultraviolet lamp to observe the influence of different concentrations of barium ions on the color reaction of the test paper. Along with the continuous increase of barium ion concentration, detection test paper color changes gradually among the present invention, thus shows that carbon dot fluorescence intensity changes gradually along with the concentration of barium ion continuous increase, and the color of detection so...
Embodiment 3
[0028] The detection of barium ion in embodiment 3 river water samples
[0029] Collect water samples to be tested S3.1~S3.4: Collect water samples with sample collection bottles at the same depth at different locations in the river, and place them for testing. Repeat the method used in Example 2 to measure aqueous solutions of known concentrations.
[0030] result
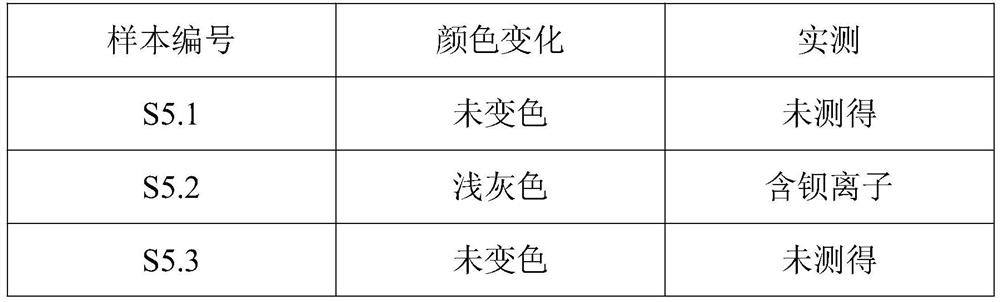
[0031] Observe the color change of the measured fluorescent test paper to determine whether the sample contains barium ions (see Table 1).
[0032] Table 1
[0033] sample number Color changes Measured S3.1 Undiscolored not measured S3.2 Undiscolored not measured S3.3 light blue Contains barium ions S3.4 Undiscolored not measured
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com