A kind of synthetic method of cyclophosphamide
The technology of a cyclophosphamide and a synthesis method is applied in the synthesis field of cyclophosphamide, can solve the problems of unfavorable industrial production, complicated reaction steps, low reaction efficiency and the like, and achieves the effects of low cost, easy operation and improved reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
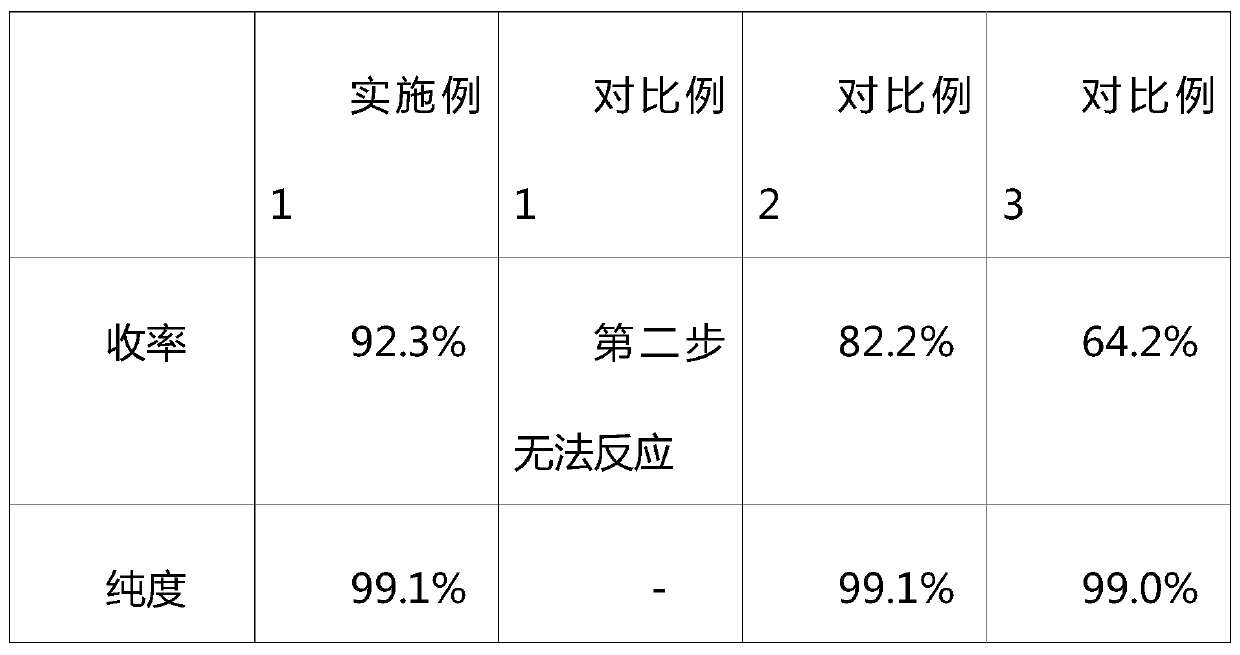
Examples
Embodiment 1
[0027] A synthetic method for cyclophosphamide, said method comprising the steps of:
[0028] (1) Slowly add 20g of phosphorus oxychloride to 100g of mixed solution of dichloroethane, polyphosphoric acid and acetic anhydride in a mass ratio of 2:2:1, add 10g of 3-aminopropanol dropwise, and react at 20°C for 0.5 hours , poured into water to separate the organic phase, washed twice with saturated aqueous sodium carbonate solution, and concentrated to obtain 2-chloro-2-oxo-[1.3.2]oxazaphosphorinane;
[0029] (2) Get 10g of 2-chloro-2-oxo-[1.3.2]oxazaphosphorinane into a pressure reaction flask, add 50g of dichloroethane and 10g of 5a molecular sieve (activated at 300°C Use after treatment), feed ammonia gas, keep at 4 atmospheres, heat to 120°C, react for 2 hours, and the reaction is complete;
[0030] (3) After the reaction liquid is filtered, add ice-water mixture, after stirring for 30 minutes, separate the organic phase, add 10% hydrochloric acid solution for washing, separ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com