The preparation method of azimidone
A technology for mezotrione and triazinon is applied in the field of preparation of mezotrione, can solve problems such as being difficult to remove, and achieve the effects of ensuring product purity, improving reaction selectivity, and improving reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
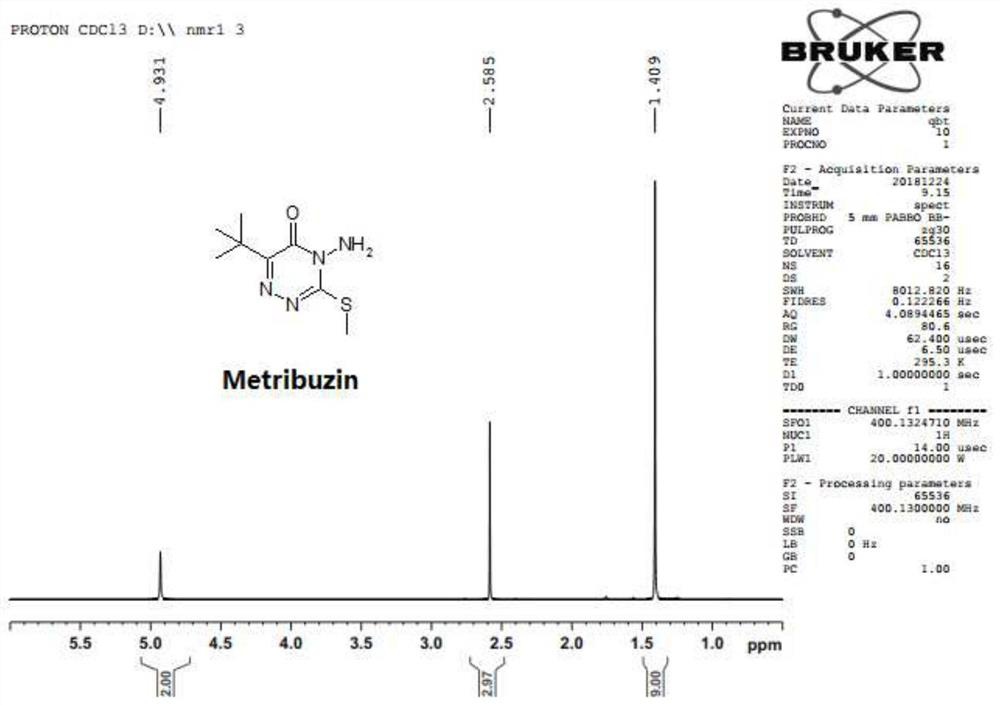
[0034] The preparation method of the namazone of the present embodiment includes the following steps:
[0035] Mechanical stirring is installed on 1000 ml of four bottles, and the air-conditioned guiding tube, thermometer and condenser are not entered. 21 g (0.88 mol) of lithium hydroxide solids and 600 ml of water were added to a four-mouth bottle, and after stirring for lithium hydroxide, 200 ml of methanol was added to the bottle.
[0036] Control the temperature of 15 ° C to 20 ° C in the bottle, slowly add 160 g (0.8 mol) triazide (4-amino-6-tert-butyl-3-thiol-1,2,4-triazine -5) with stirring. Ketone), stirred and dissolved.
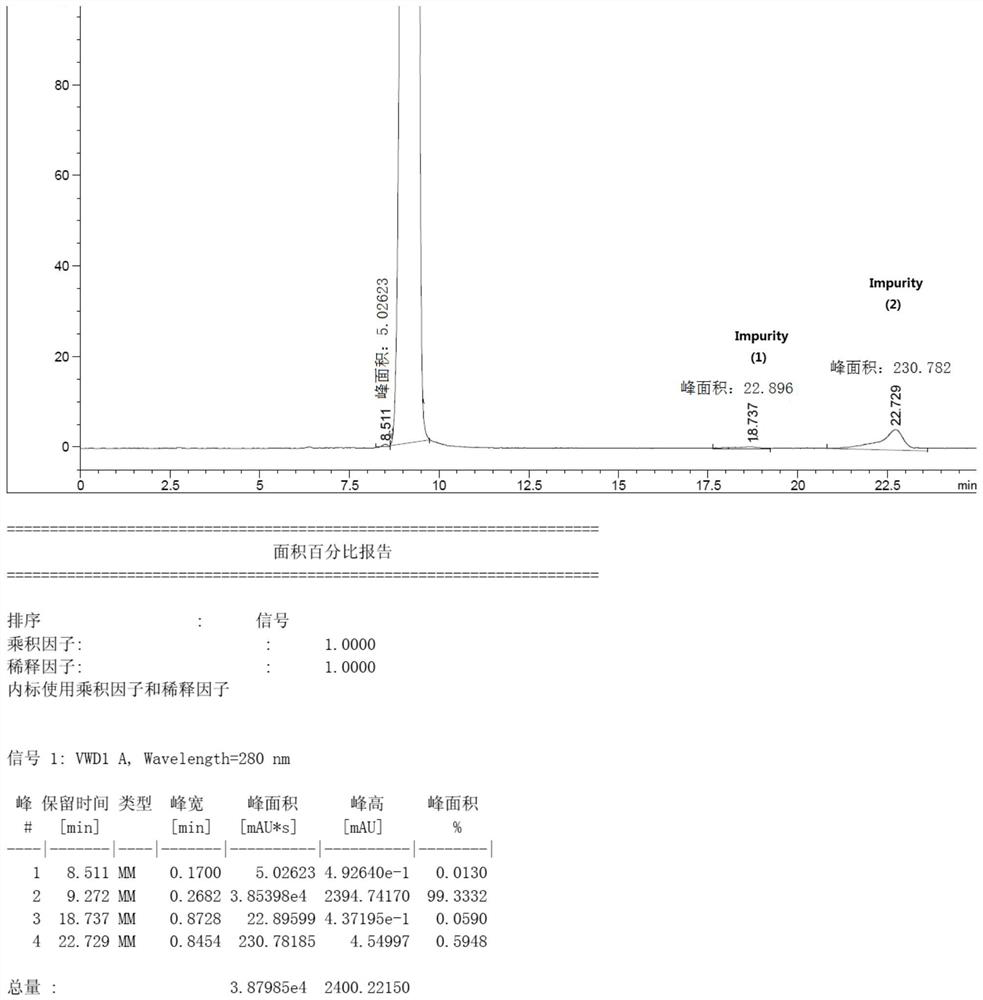
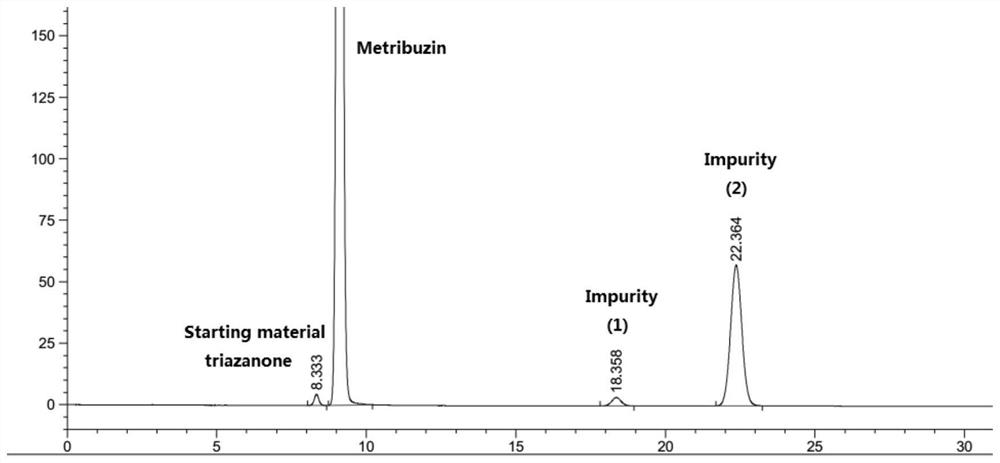
[0037] The bromomethane gas was introduced into the bottle, and the lithium hydroxide solution of 2 mol / L was added dropwise as the passage of bromomethane gas was slowly added dropwise to maintain a pH of the reaction system of 11.5 to 12.5, and liquid chromatography was tracked. The triazinone completely disappears (the reaction ends in this example...
Embodiment 2)
[0049] The preparation method of the namazone of the present embodiment includes the following steps:
[0050] Mechanical stirring is installed on 1000 ml of four bottles, and the air-conditioned guiding tube, thermometer and condenser are not entered. Was added solid lithium hydroxide and 21g water 600mL four-neck flask, lithium hydroxide was stirred until complete dissolution, methanol was added 200mL bottle.
[0051] Controlling inner temperature 15 ℃ ~ 20 ℃, under stirring slowly added 160g triazin-one (4-amino-6-tert-butyl-3-mercapto-1,2,4-triazin-5-one), stirred a clear solution.
[0052] Was slowly passed through the chloride gas bottle, and passed as methyl chloride gas was slowly added dropwise 2 mol / L lithium hydroxide solution to maintain the pH value of the reaction system, 11.5 to 12.5, with the sample liquid chromatography tracking complete disappearance of the triazine-one (Example 14 of the present embodiment the end of the reaction to 16 hours); inner temperatur...
Embodiment 3)
[0055] The preparation method of the namazone of the present embodiment includes the following steps:
[0056] Mechanical stirring is installed on 1000 ml of four bottles, and the air-conditioned guiding tube, thermometer and condenser are not entered. 34g solid sodium hydroxide, lithium 37g (0.87 mol) solid chloride and water were added 600mL four-necked flask, stirring until completely dissolved, methanol was added 200mL bottle.
[0057] Controlling inner temperature 10 ℃ ~ 15 ℃, was slowly added with stirring 160g triazin-one (4-amino-6-tert-butyl-3-mercapto-1,2,4-triazin-5-one), stirred a clear solution.
[0058] Slowly to the methyl bromide gas into the bottle, and with the methyl bromide gas is passed slowly added dropwise 2 mol / L sodium hydroxide solution, the reaction system to maintain a pH value of 11.5 to 12.5, with the sample tracking liquid chromatography complete disappearance of the triazine-one (Example 5 of the present embodiment the end of the reaction to 6 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com