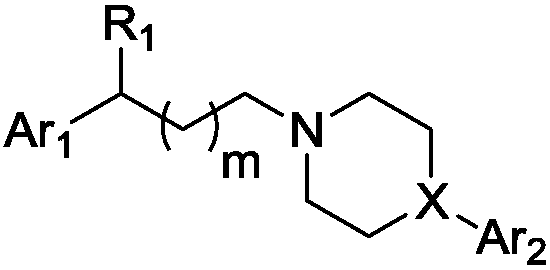
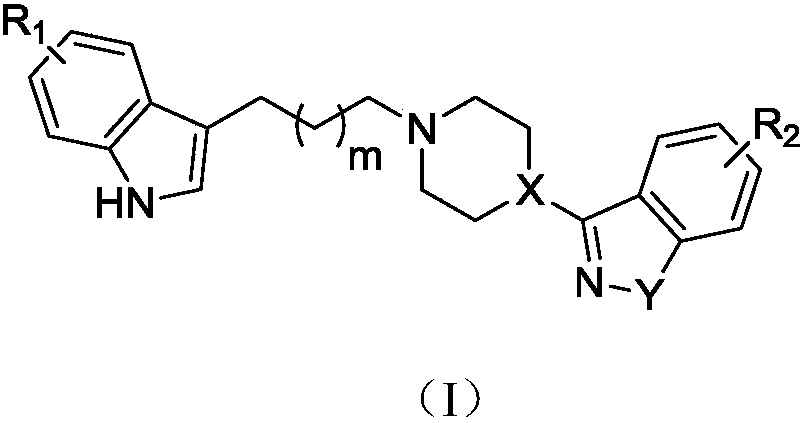
Indole-heteroaromatic piperazine (piperidine) derivatives and application thereof in resisting depression
A technology of aromatic heterocycles and indole, which is applied in the fields of new drug design, synthesis and discovery, can solve the problems of inability to improve cognitive impairment, large side effects, and low curative effect, and achieve ideal pharmacokinetic characteristics, small side effects, and wide indications Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Preparation of 3-(4-(2-(5-fluoro-1H-indol-3-yl)ethyl)piperazin-1-yl)benzo[d]isothiazole (VI-1) hydrochloride
[0072] 5-Fluoro-1H-indole (I) (0.1 mol) and AlCl 3 (0.1mol) was dissolved in dichloromethane (200mL), the temperature was lowered to 0°C, and a dichloromethane solution (50mL) of chloroacetyl chloride (II) (0.1mol) was slowly added dropwise with stirring. According to General Method 1, 14.81 g of Intermediate III (colorless liquid) was obtained with a yield of 70%. MS (m / z): 212.03[M+1] + .
[0073] The above intermediate (III) (0.05mol) was dissolved in trifluoroacetic acid (50mL), cooled to 0°C, and HSiEt was slowly added dropwise under stirring 3 (0.15 mol). According to the operation of General Method 2, 6.92 g of intermediate IV (colorless liquid) was obtained, and the yield was 70%. MS(m / z): 198.16[M+1] + .
[0074] Above-mentioned intermediate IV (0.01mol) and the hydrochloride (0.01mol) of piperazine compound (V) are dissolved in DMF (100mL), add...
Embodiment 2
[0076] Preparation of 3-(4-(3-(5-fluoro-1H-indol-3-yl)propyl)piperazin-1-yl)benzo[d]isothiazole (VI-2) hydrochloride
[0077] 5-Fluoro-1H-indole (I) (0.1 mol) and AlCl 3 (0.1mol) was dissolved in dichloromethane (200mL), the temperature was lowered to 0°C, and a dichloromethane solution (50mL) of 3-chloropropionyl chloride (II) (0.1mol) was slowly added dropwise under stirring. According to General Method 1, 13.54 g of Intermediate III (colorless liquid) was obtained with a yield of 60%. MS(m / z): 226.05[M+1] + .
[0078] The above intermediate (III) (0.05mol) was dissolved in trifluoroacetic acid (50mL), cooled to 0°C, and HSiEt was slowly added dropwise under stirring 3 (0.15 mol). According to the operation of General Method 2, 6.35 g of intermediate IV (colorless liquid) was obtained, and the yield was 60%. MS (m / z): 212.04[M+1]+ .
[0079] Above-mentioned intermediate IV (0.01mol) and the hydrochloride (0.01mol) of piperazine compound (V) are dissolved in DMF (100mL)...
Embodiment 3
[0081] Preparation of 3-(4-(4-(5-fluoro-1H-indol-3-yl)butyl)piperazin-1-yl)benzo[d]isothiazole (VI-3) hydrochloride
[0082] 5-Fluoro-1H-indole (I) (0.1 mol) and AlCl 3 (0.1mol) was dissolved in dichloromethane (200mL), cooled to 0°C, and a dichloromethane solution (50mL) of 4-chlorobutyryl chloride (II) (0.1mol) was slowly added dropwise under stirring. According to General Method 1, 20.37 g of Intermediate III (colorless liquid) was obtained, with a yield of 85%. MS(m / z): 240.15[M+1] + .
[0083] The above intermediate (III) (0.05mol) was dissolved in trifluoroacetic acid (50mL), cooled to 0°C, and HSiEt was slowly added dropwise under stirring 3 (0.15 mol). According to the operation of General Method 2, 9.03 g of intermediate IV (colorless liquid) was obtained, and the yield was 80%. MS (m / z): 226.14[M+1] + .
[0084] Above-mentioned intermediate IV (0.01mol) and the hydrochloride (0.01mol) of piperazine compound (V) are dissolved in DMF (100mL), add K 2 CO 3 (0.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com