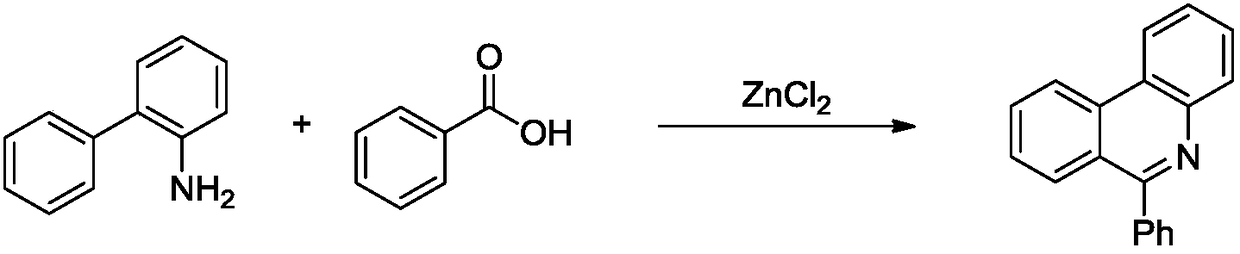
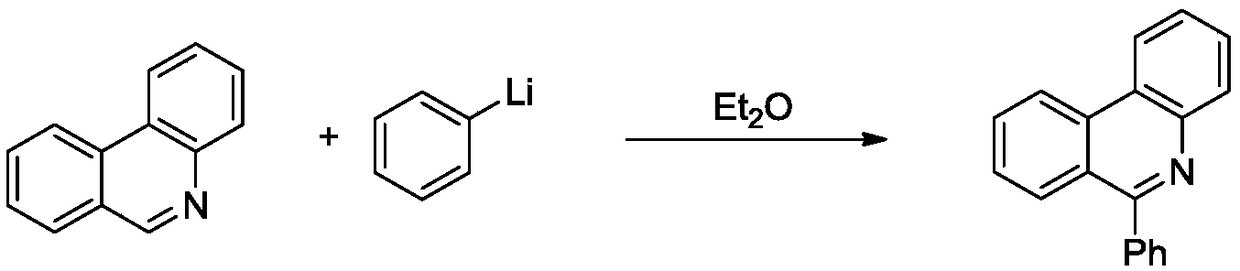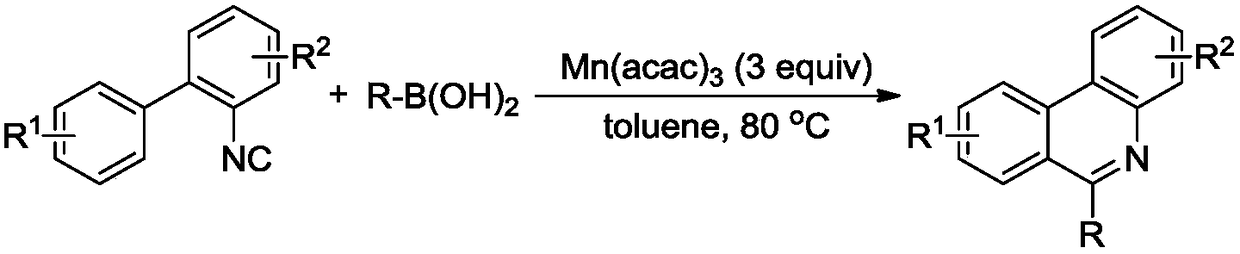6-(alpha-cyanoimine) based phenidine compound and synthesis method thereof
A cyanoimine and compound technology, applied in the field of 6-phenanthridine compounds and their synthesis, can solve problems such as limited types of compounds, and achieve the effects of good application prospects, simple raw materials and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: (Z)-N-(tert-butyl)phenanthridine-6-methanimine cyanide
[0044] (Z)-N-(tert-butyl)phenanthridine-6-methylimine cyanide adopts the following steps: 1. add 15.9 grams of N-p-toluenesulfonyl-5,6-dihydrophenanthrene in a 1000 ml reactor Pyridine, 20.1 ml tert-butylisonitrile, 1.74 g silver triflate, 30.6 g 2,3-dichloro-5,6-dicyano-1,4-benzoquinone, 675 ml chlorobenzene, heated to 80 ℃. Use thin layer chromatography to track the reaction until the reaction raw materials disappear; ② After the reaction, extract the product with ethyl acetate, wash with saturated brine, and remove the solvent with a rotary evaporator after drying to obtain a crude product; ③ Use a column layer for the crude product Analyze (petroleum ether: ethyl acetate=20:1) purify, obtain 8.8 gram (Z)-N-(tert-butyl)phenanthridine-6-methanimine cyanides, its structural formula is: The yield was 68%. Melting point: 103-105°C.
[0045] IR(KBr,cm -1 ):3075,2975,2217,1612,1450,1362,1207,937;
[0...
Embodiment 2
[0050] Example 2: (Z)-N-(tert-butyl)-8-methylphenanthridine-6-methanimine cyanide
[0051] (Z)-N-(tert-butyl)-8-methylphenanthridine-6-methylimine cyanide adopts the following steps: 1. add 15.6 grams of N-p-toluenesulfonyl-5 in 1000 milliliters of reaction kettle, 6-dihydro-8-methylphenanthridine, 25.2 ml tert-butylisonitrile, 1.74 g silver triflate, 15.9 g 2,3-dichloro-5,6-dicyano-1,4-benzoquinone , 450 ml of chlorobenzene, heated to 90°C. Use thin layer chromatography to track the reaction until the reaction raw materials disappear; ② After the reaction, extract the product with ethyl acetate, wash with saturated brine, and remove the solvent with a rotary evaporator after drying to obtain a crude product; ③ Use a column layer for the crude product Analyze (petroleum ether: ethyl acetate=20:1) purify, obtain 7.18 grams of (Z)-N-(tert-butyl)-8-methylphenanthridine-6-methanimine cyanide, its structural formula is: The yield was 53%. Melting point: 158-160°C.
[0052] IR(...
Embodiment 3
[0057] Example 3: (Z)-8-bromo-N-(tert-butyl)phenanthridine-6-methanimine cyanide
[0058] (Z)-N-(tert-butyl)-8-bromophenanthridine-6-methylimine cyanide adopts the following steps: ①add 18.6 grams of N-p-toluenesulfonyl-5,6 in a 1000 ml reaction kettle -Dihydro-8-bromophenanthridine, 20.1 ml tert-butylisonitrile, 1.74 g silver triflate, 30.6 g 2,3-dichloro-5,6-dicyano-1,4-benzoquinone, 450 mL of chlorobenzene, heated to 85°C. Use thin layer chromatography to track the reaction until the reaction raw materials disappear; ② After the reaction, extract the product with ethyl acetate, wash with saturated brine, and remove the solvent with a rotary evaporator after drying to obtain a crude product; ③ Use a column layer for the crude product Analyze (petroleum ether: ethyl acetate=20:1) purify, obtain 5.18 gram (Z)-N-(tert-butyl)-8-methylphenanthridine-6-methanimine cyanides, its structural formula is: The yield was 31%. Melting point: 172-174°C.
[0059] IR(KBr,cm -1 ):2967.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com