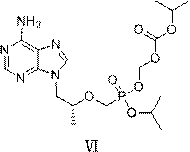
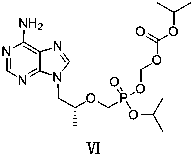
Tenofovir disoproxil fumarate analog preparation method
A technology of tenofovir fumarate and dipivoxil, which is applied in the field of medicinal chemistry and can solve problems such as unsuitable for large-scale preparation, large amount of organic solvents, and large pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
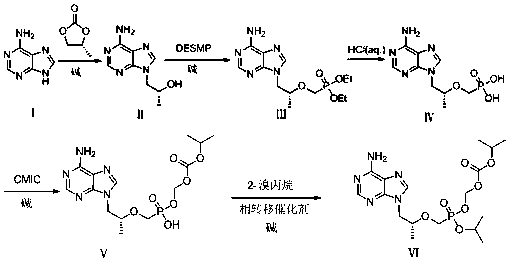
[0053] first step:( R )-1-(6-amino-9H-purin-9-yl)propyl-2-alcohol (Ⅱ) preparation
[0054] Add 80.00g (0.592mol) of adenine into a three-necked flask, add 380ml of N,N-dimethylformamide (DMF), add 9.48g (0.237mol) of sodium hydroxide under stirring, and then add ( S )-propylene carbonate 78.60g (0.770mol), heat up to 130°C, react for 18 hours, stop the reaction, cool down to 40°C, add a mixed solution of methanol 240ml and isopropanol 240ml, cool down to 12°C, constant temperature crystallization 1 hours, filtered, and the filter cake was washed with a mixed solution (4°C) of 40ml of methanol and 40ml of isopropanol, drained, and dried in a vacuum oven at 45°C for 4.5 hours to obtain 87.70g (0.454mol) of a white solid, with a molar yield of was 76.7%.
[0055] 1 H NMR (400 MHz, DMSO- d 6 ): δ = 8.15 (s, 1H), 8.05 (s, 1H), 7.18 (s,2H), 5.03 (d, J = 4.0 Hz, 1H), 4.11 (q, J = 7.4 Hz, 1H), 4.07 – 3.98 (m, 2H), 1.07 (d, J = 5.8 Hz, 3H) ppm.
[0056] 13 C NMR (100 MHz, ...
Embodiment 2
[0061] first step:( R )-1-(6-amino-9H-purin-9-yl)propyl-2-alcohol (Ⅱ) preparation
[0062] Add 40.00g (0.296mol) of adenine into a three-necked flask, add 190ml of N,N-dimethylformamide (DMF), add 0.94g (0.0235mol) of sodium hydroxide under stirring, and then add ( R )-propylene carbonate 39.20g (0.384mol), heat up to 120°C, react for 27 hours, stop the reaction, cool down to 70°C, add a mixed solution of methanol 120ml and isopropanol 120ml, cool down to 15°C, constant temperature crystallization 12 hours, filtered, the filter cake was washed with a mixed solution of methanol 20ml and isopropanol 20ml (4°C), drained, and placed in a vacuum oven at 60°C for 2 hours to obtain 45.51g (0.215mol) of white solid, molar yield was 72.6%.
Embodiment 3
[0064] The second step: ( R )-diethyl (((1-(6-amino-9H-purin-9-yl) propyl-2-ol) oxygen) methyl) phosphate (Ⅲ) preparation
[0065] Add 40.00g (0.207mol) of intermediate (Ⅱ) and N-methylpyrrolidone (NMP) (160ml) into a dry three-necked flask and stir to dissolve. o Add 70.00g (0.414mol) of magnesium tert-butoxide to C, then raise the temperature to 70 o C, then slowly add 100.00g (0.311mol) of (diethoxyphosphono)methyl-4-methylbenzenesulfonate, react at a constant temperature of 70°C for 4h, then cool down to 20±5°C, and slowly add 36.75g (0.612mol) of acetic acid was used to adjust the pH to 6-7, and the temperature was kept at 15-25°C. Add 900ml of ethyl acetate to the mixture, stir for 30min, let stand, and pour out the supernatant. Put the filter cake in a bottle, add 300ml of ethyl acetate, stir at 15-25°C for 30 minutes, filter, combine with the first filtrate, filter the combined filtrate again, concentrate the filtrate under reduced pressure to obtain a light yellow o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com