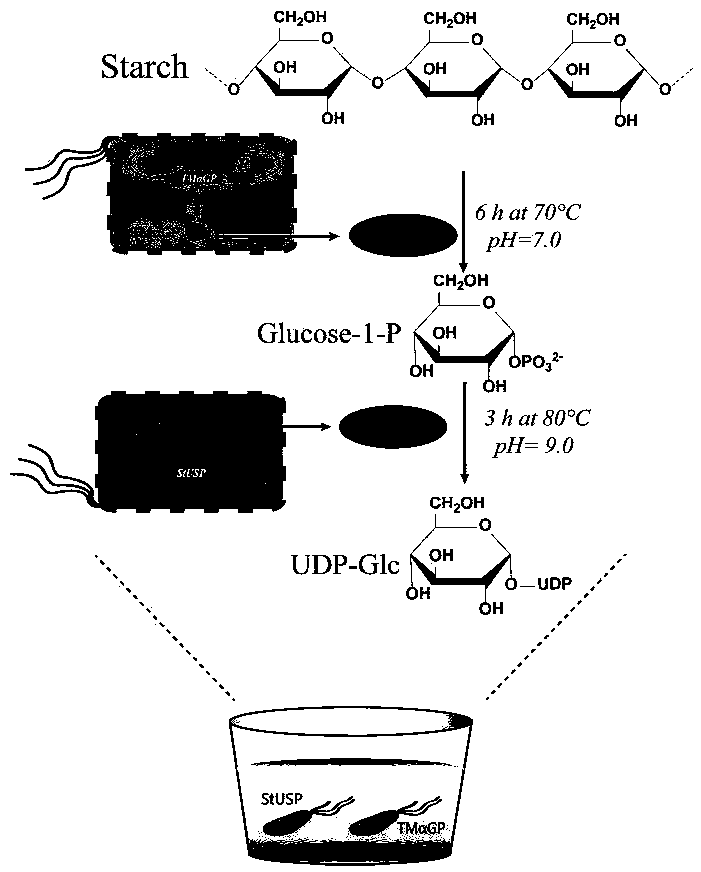
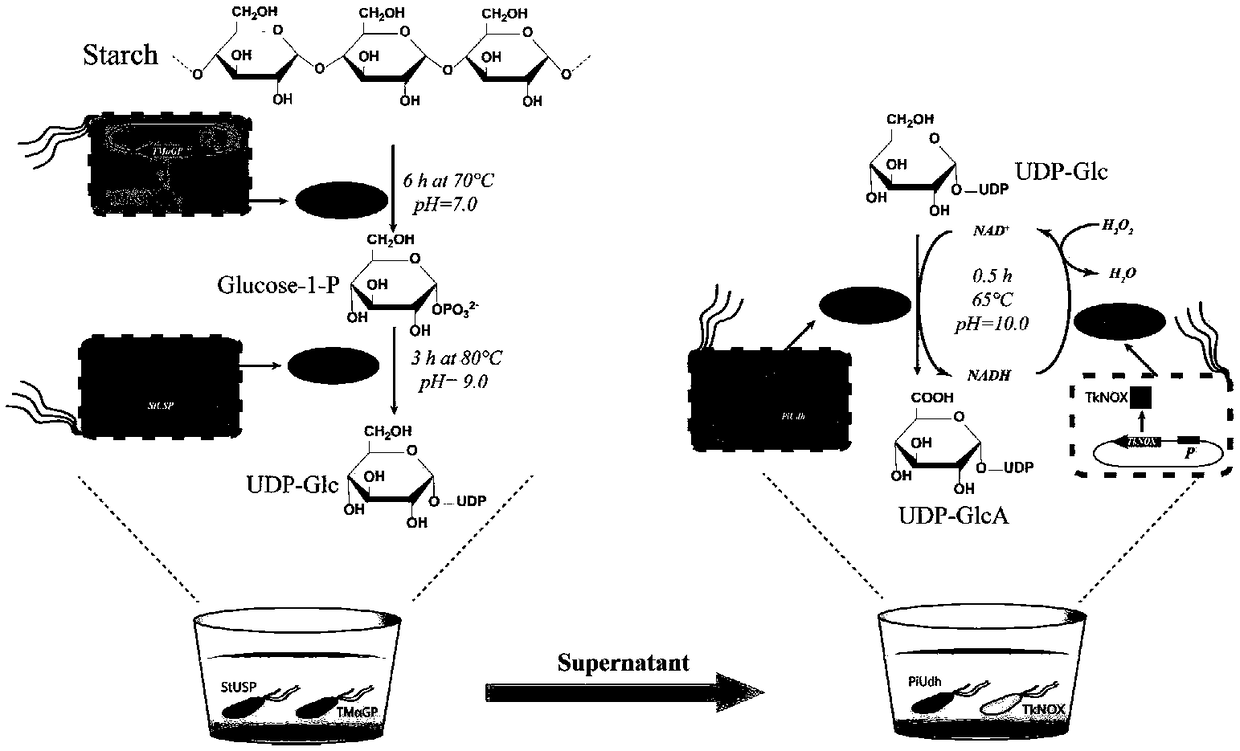
Biosynthesis method of uridine diphosphate glucose and uridine diphosphate glucuronic acid
A technology for uridine diphosphate glucose and biosynthesis, which is applied in the field of biosynthesis of uridine diphosphate glucose and uridine diphosphate glucuronic acid, can solve the problems of complex preparation steps and high cost of raw materials, and achieves simplified operation steps and economical savings. The effect of time and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
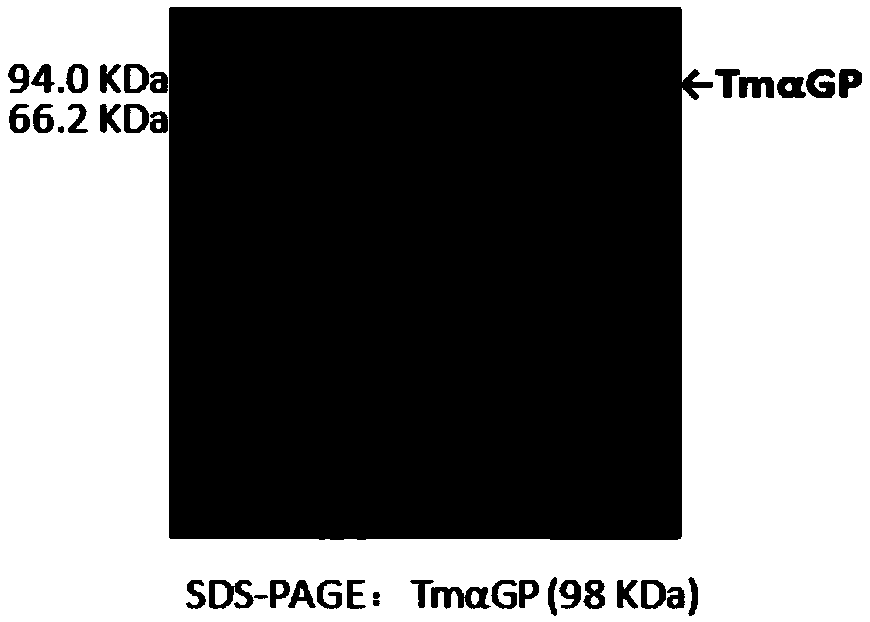
[0096] Example 1. Induced expression of recombinant Escherichia coli high temperature α-glucan phosphorylase (TmαGP) and high temperature sugar-1-phosphate nucleotidylase (StUSP)
[0097] Inoculate the recombinant Escherichia coli BL21 (DE3) containing the target gene TmαGP or StUSP synthesized by GenScript into 30 mL of LB medium (containing 100 μg / mL of ampicillin), and activate at 225 rpm for 12 to 14 hours at 37°C; then , the activated recombinant Escherichia coli BL21 (DE3) was inoculated in 250 mL of LB medium (containing 100 μg / mL of ampicillin) for expansion culture, and the initial OD 600 The value is 0.05, cultured to OD at 37°C 225rpm 600 When the value reaches 0.6-0.8, add IPTG with a final concentration of 0.2mM to induce, the induction condition is 22°C 225rpm, 18-20h, measure the OD of the recombinant E. coli liquid 600 Value, wherein, the OD after induced expression of recombinant Escherichia coli containing the target gene TmαGP 600 The value is 2.1-2.3; the...
Embodiment 2
[0099] Example 2. High-temperature whole-cell catalytic reaction of high-temperature α-glucan phosphorylase (TmαGP)
[0100] 1) High-temperature whole-cell catalytic reaction of TmαGP
[0101] First, centrifuge the recombinant Escherichia coli bacterium containing induced expression TmαGP prepared in Example 1 to collect the bacterial pellet, freeze the obtained bacterial pellet at -20°C for about one day, take it out and melt it on ice during the reaction, and use 60 mL of bacteria The bacterial pellet obtained by liquid centrifugation was resuspended in 2 mL triple distilled water. The reaction system is shown in Table 4, wherein the KP solution is KH 2 PO 4 and K 2 HPO 4 Prepared by dissolving in water, KH in KP solution 2 PO 4 and K 2 HPO 4 Concentrations of 0.38M and 0.62M, pH 7, PO 4 3- The total concentration of TmαGP cells is 1M, and the amount of TmαGP cells added is 6.6g cell dry weight / L reaction solution; in addition, a control group is set up, respective...
Embodiment 3
[0114] Example 3. High-temperature whole-cell catalytic reaction of high-temperature sugar-1-phosphate nucleotidylase (StUSP)
[0115] 1) High-temperature whole-cell catalytic reaction of StUSP
[0116] First, centrifuge the recombinant Escherichia coli liquid containing induced StUSP prepared in Example 1 to collect the bacterial precipitate, freeze the obtained bacterial precipitate at -20°C for about one day, take it out and melt it on ice during the reaction, and use 60mL bacterial The bacterial pellet obtained by liquid centrifugation was resuspended in 2 mL triple distilled water. The reaction system is shown in Table 5, wherein, Mg 2+ Produced by the hydrolysis of magnesium chloride, the component of sodium dihydrogen phosphate buffer is NaH 2 PO 4 0.2M, NaOH to adjust the pH to 7.5; in addition, set up a control group, respectively, no StUSP cells in the reaction solution, and uridine diphosphate glucose (UDP-Glc) in the reaction solution, each reaction solution wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com