S77 mutant protein of osteoprotegerin, as well as relevant product and application thereof
A technology of osteoprotegerin and related products, applied in the fields of application, peptide/protein composition, animal/human protein, etc., can solve problems such as increasing tumor risk and affecting clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
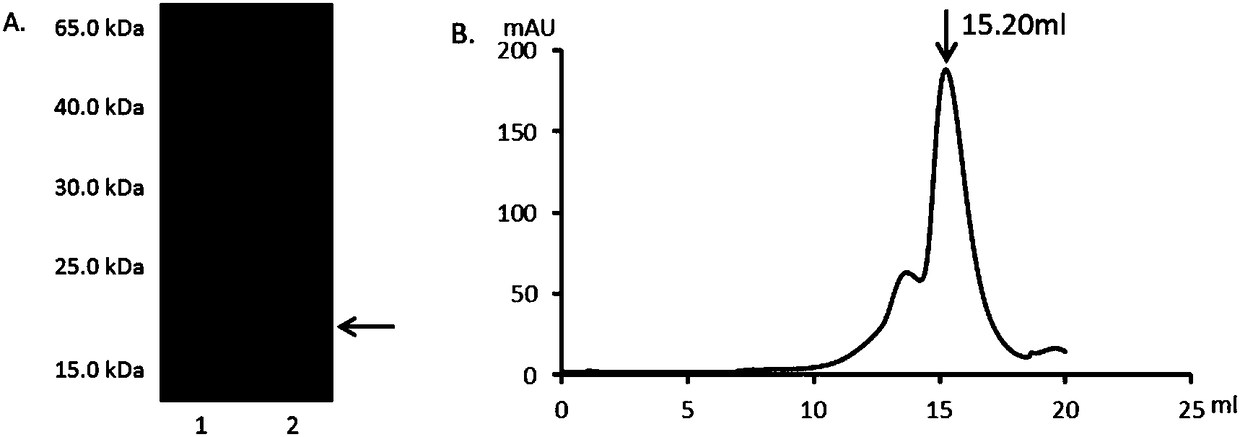
[0108] Example 1. Preparation of wild-type human osteoprotegerin OPGwt and wild-type human osteoprotegerin mutant proteins OPGS77G, OPGS77D, OPGS77V, OPGS77F, OPGS77E, OPGK108A and OPGR122G
[0109] 1. Preparation of wild-type human osteoprotegerin OPGwt
[0110] The amino acid sequence of wild-type human osteoprotegerin OPGwt is the 154th to 345th amino acid residues of SEQ ID No.2. Wild-type human osteoprotegerin OPGwt contains amino acid residues 22-201 of human full-length osteoprotegerin (amino acid sequence is GenBank AccessionNumber NP_002537.3 (Update Date: 26-JUN-2017) or SEQ ID No.10)).
[0111] Wild-type human osteoprotegerin OPGwt was prepared according to the method of the following literature: Mengmeng Jin, et al. Sortase A-aided Escherichia coli expression system for functional osteoprotegerin cysteine-rich domain. Appl Microbiol Biotechnol (2017) 101:4923–4933. DOI10.1007 / s00253 -017-8188-6. The specific method is as follows:
[0112] 1.1 Preparation of DNA ...
Embodiment 2
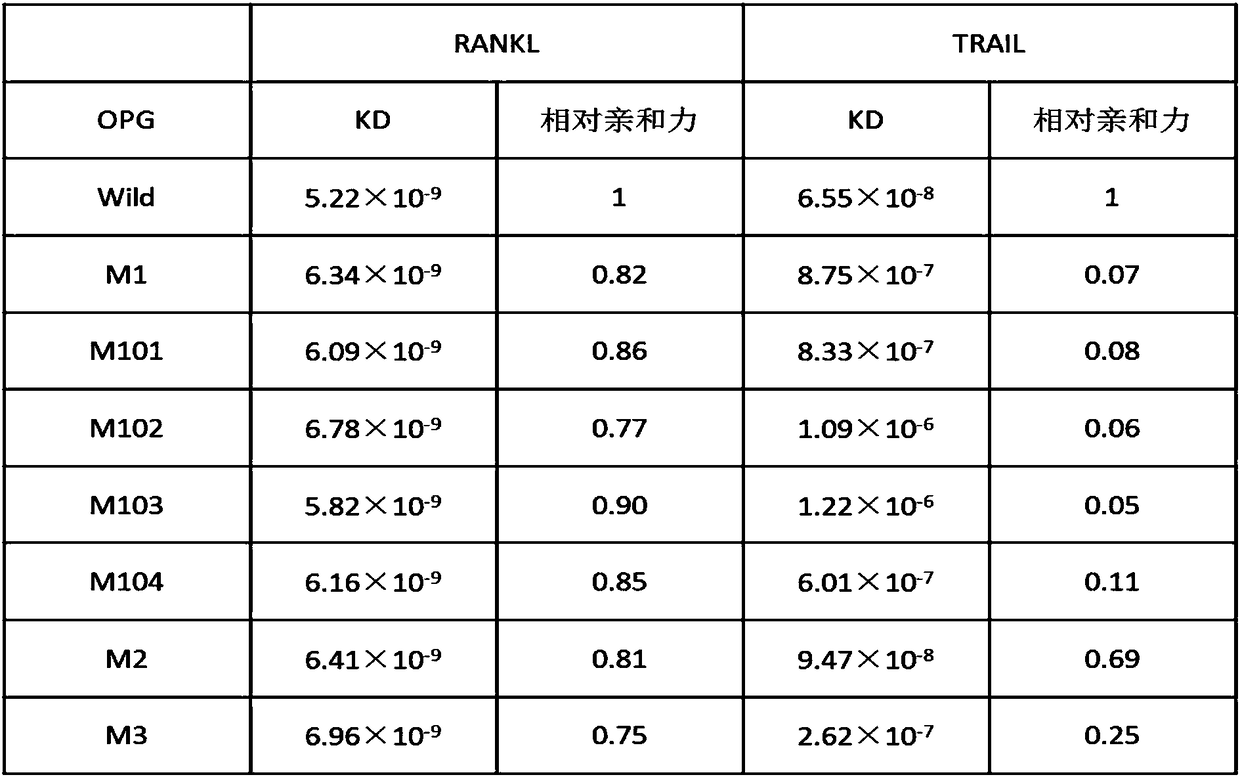
[0178] Example 2, Affinity Determination of Wild-type Human Osteoprotegerin OPGwt and Human Osteoprotegerin Mutant Proteins OPGS77G, OPGS77D, OPGS77V, OPGS77F, OPGS77E, OPGK108A and OPGR122G with RANKL and TRAIL
[0179] The surface plasmon resonance (Surface Plasmon Resonance, SPR) technology was used, and the equipment used was BIAcore 3000 (GE Healthcare), and RANKL or TRAIL was used as the stationary phase to immobilize on a certain channel on the surface of the CM5 chip (diluted into 10mM sodium acetate pH5.5 ), the response value increased by about 2000RU is better; TNFRSF9 was fixed in another channel as an internal reference; OPGwt prepared in Example 1 and mutant proteins OPGS77G, OPGS77D, OPGS77V, OPGS77F, OPGS77E, OPGK108A and OPGR122G were mobile phases , OPGwt and human osteoprotegerin mutant proteins OPGS77G, OPGS77D, OPGS77V, OPGS77F, OPGS77E, OPGK108A and OPGR122G were prepared in different concentrations (0, 3.125, 6.25, 12.5, 25, 50, 100nM), flow through the ...
Embodiment 3
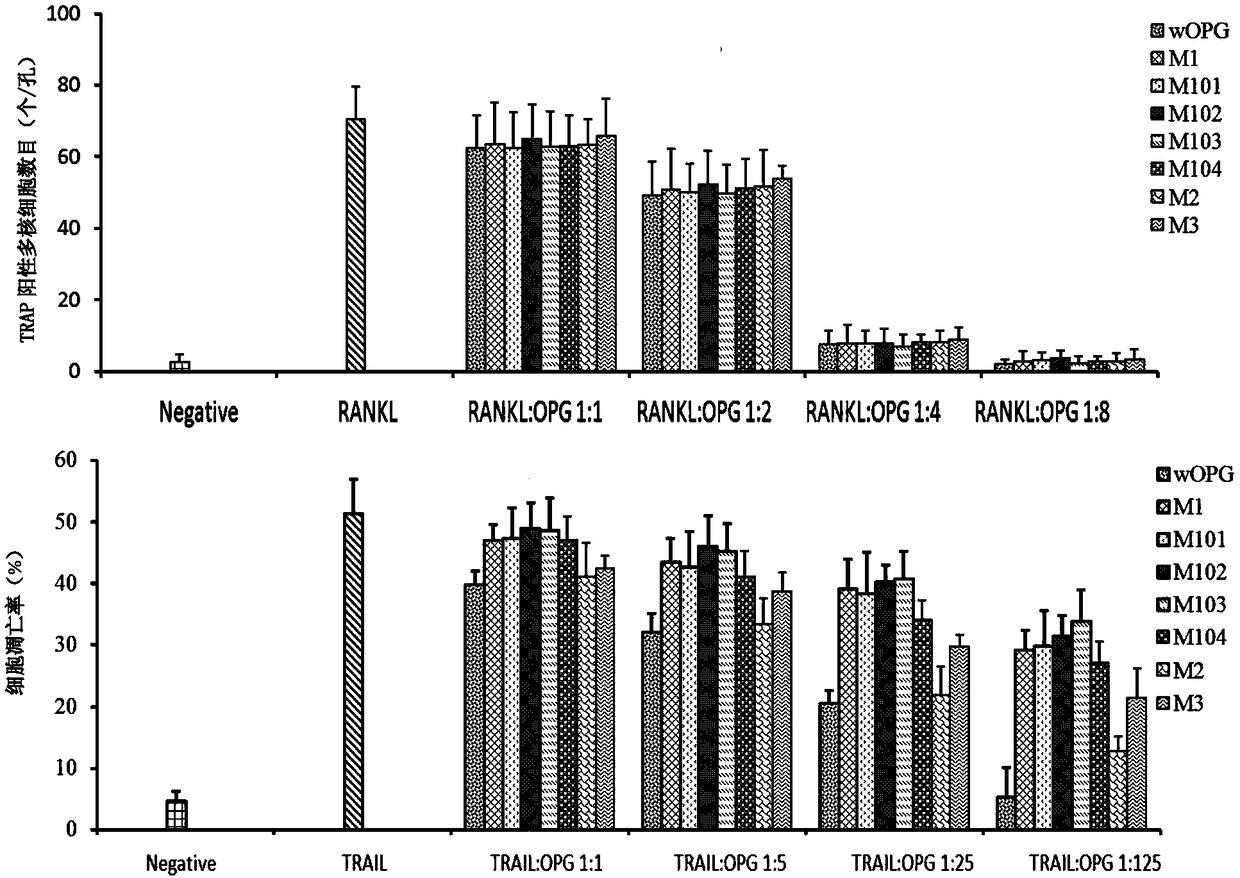
[0191] Example 3, Biological activity of wild-type human osteoprotegerin OPGwt and mutant proteins OPGS77G, OPGS77D, OPGS77V, OPGS77F, OPGS77E, OPGK108A and OPGR122G of human osteoprotegerin
[0192] 1. Inhibition of RANKL-mediated osteoclast activation experiment
[0193] The differentiation and maturation of RANKL-induced osteoclasts are marked by the formation of multinucleated cells and the expression of tartrate-resistant acid phosphatase (TRAP) in multinucleated cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com