Preparation method of mannuronate oligosaccharides, and application of mannuronate oligosaccharides in preparation of combined antibacterial agent for inhibiting drug-resistant bacteria
A technology of uronic acid oligosaccharides and antibacterial agents, which is applied in the field of medicine, can solve the problems of high cost, complicated operation, and low purity, and achieve the effects of increasing potency, simple preparation method, and reducing MIC value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] a. Take 15g of kelp, add 200mL of formaldehyde hydrochloric acid solution (10mL of formaldehyde plus 0.84mL of hydrochloric acid water to make up to 100mL, shake well), soak for 4h, wash with water, and filter with gauze. Add 600mL of 1% sodium carbonate solution to the filter residue, bathe in 60°C water for 3h, let cool, add 100mL of water, stir evenly, centrifuge at 4000rpm×5min, and take the supernatant. The pH of the supernatant was adjusted to 2.0 with hydrochloric acid and allowed to stand for 12 hours. Collect the gel by centrifugation at 4000rpm×5min. Add 200mL of 2% sodium hydroxide solution to the gel, stir to dissolve the gel completely, add 400mL of ethanol, mix well, and precipitate with alcohol to obtain a yellow-white gel-like precipitate (algin), which should be lyophilized and stored away from light.
[0031] b. Take 2g of alginate, add 400mL of water, and soak overnight. Add an appropriate amount of hydrochloric acid to make the concentration 0.2mol...
Embodiment 2
[0037] a, with embodiment 1.
[0038] b. Take 2g of alginate, add 400mL of water, and soak overnight. Add an appropriate amount of hydrochloric acid to make the concentration 0.2mol / L, heat in a water bath at 100°C and stir magnetically for 4 hours. After cooling, centrifuge to take the supernatant, adjust the pH of the supernatant to neutral after rotary evaporation, and freeze-dry to obtain crude LMOS.
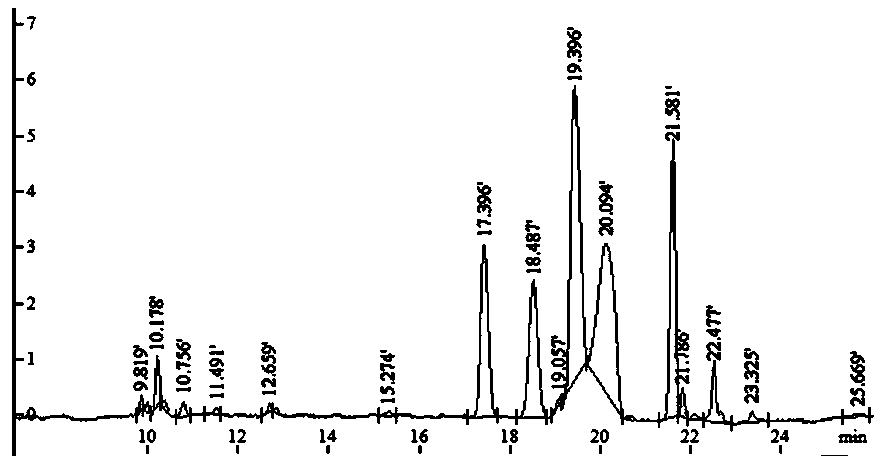
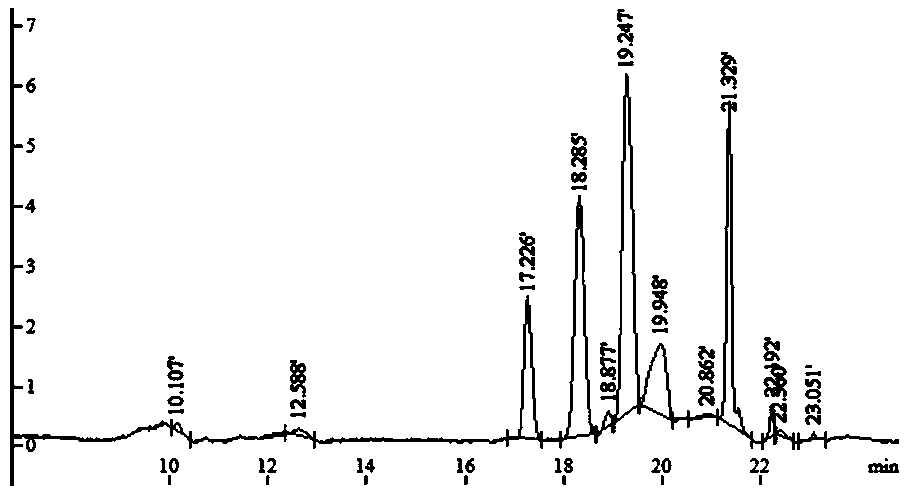
[0039] c. Dissolve an appropriate amount of the above-mentioned LMOS in 800 μL of water, vortex and mix well, and centrifuge at 12000 rpm for 3 min. Take the centrifuged supernatant and put it on a Sephadex-25 dextran gel column (2.6 cm×30 cm), use water as the eluent, collect the eluate, about 3mL per tube, use the phenol-sulfuric acid method to detect sugar, and use nitric acid The silver test solution detects salt, and water is used as a control. Collect the salt-free and sugar-containing eluate, and freeze-dry to obtain the finished LMOS. The resulting finished produ...
Embodiment 3
[0041] a, with embodiment 1.
[0042] b. Take 2g of alginate, add 400mL of water, and soak overnight. Add an appropriate amount of hydrochloric acid to make the concentration 0.1mol / L, heat in a water bath at 100°C and stir magnetically for 2h. After cooling, centrifuge to take the supernatant, adjust the pH of the supernatant to neutral after rotary evaporation, and freeze-dry to obtain crude LMOS.
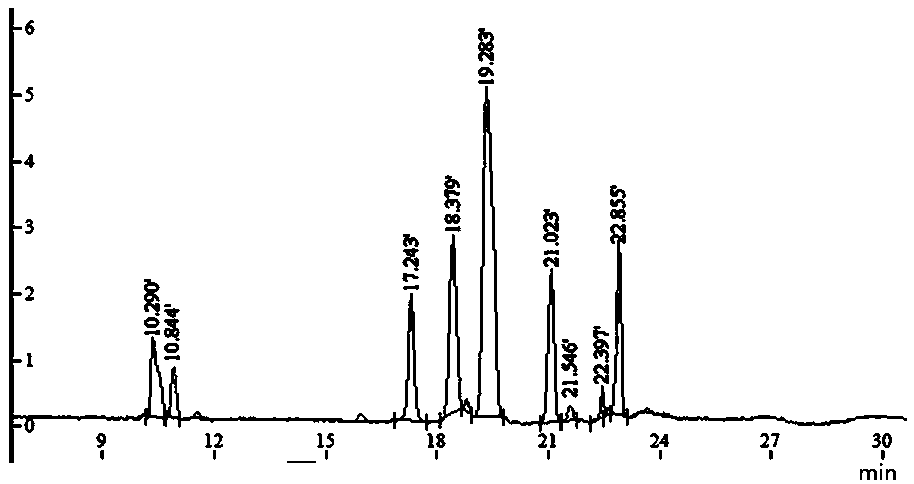
[0043] c. Dissolve an appropriate amount of the above-mentioned LMOS in 800 μL of water, vortex and mix well, and centrifuge at 12000 rpm for 3 min. Take the centrifuged supernatant and put it on a Sephadex-25 dextran gel column (2.6 cm×30 cm), use water as the eluent, collect the eluate, about 3mL per tube, use the phenol-sulfuric acid method to detect sugar, and use nitric acid The silver test solution detects salt, and water is used as a control. Collect the salt-free and sugar-containing eluate, and freeze-dry to obtain the finished LMOS. The resulting finished product is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com