A liposome composition capable of regulating drug release and its preparation method
A liposome composition and an adjustable technology, which can be applied in the directions of liposome delivery, pharmaceutical formulations, medical preparations of inactive ingredients, etc., and can solve the problems of complex reaction steps, insensitivity to acid stimulation, low cell uptake efficiency, etc. , to achieve the effects of mild conditions, reduced production costs and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
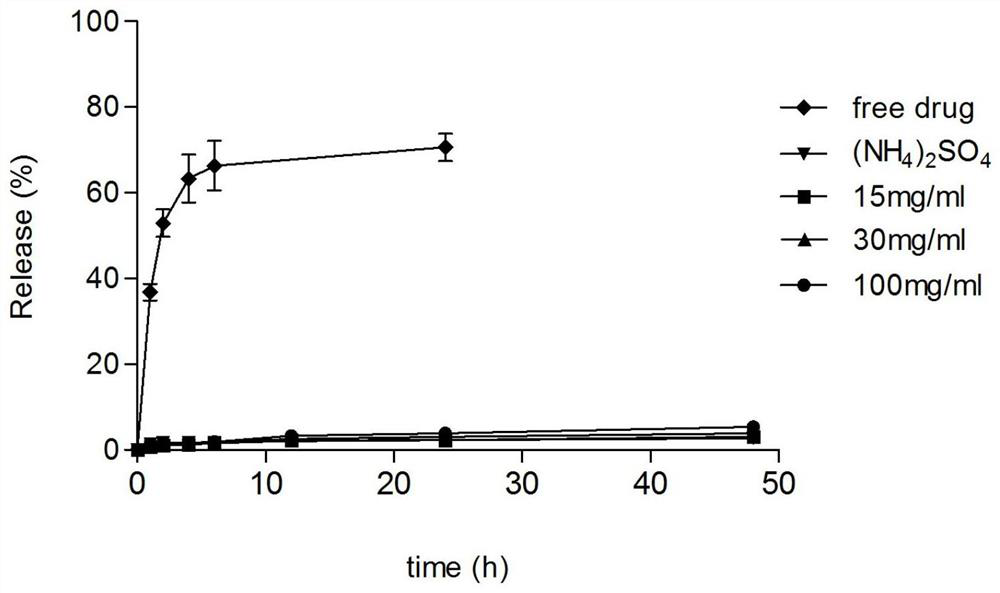
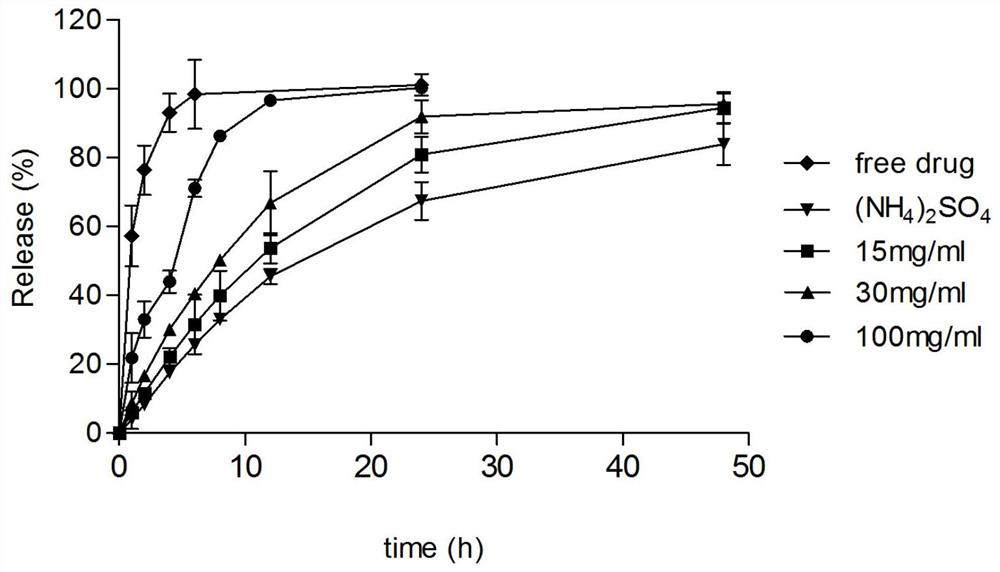
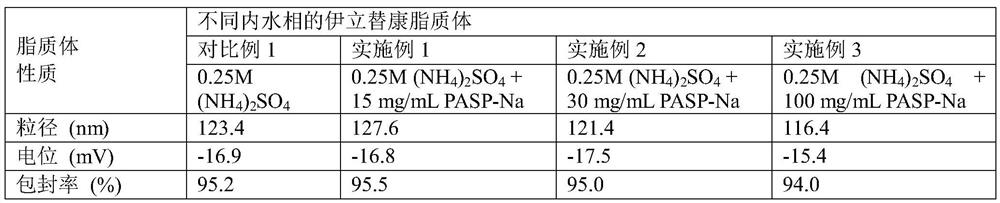
[0063] The preparation of drug-loaded liposomes containing sodium polyaspartate (15mg / mL) in the inner aqueous phase:
[0064] Preparation of blank liposomes: Dissolve distearoylphosphatidylcholine (DSPC), cholesterol, distearoylphosphatidylethanolamine-polyethylene glycol (DSPE-PEG) at a mass ratio of 3:1:0.36 in After fully dissolving in water ether, mix well after fully dissolving, then evaporate the organic solvent ether under reduced pressure, so that film-forming materials such as phospholipids form a uniform lipid film at the bottom of the flask, and then add 0.25M ammonium sulfate and 15mg / mL sodium polyaspartate (MW=9k) aqueous solution, hydration in 50°C water bath for 30 minutes, vortexed for 5 minutes, using liposome extrusion equipment, extruding the obtained liposome suspension through polycarbonate with a pore size of 200nm and 100nm membranes 10 times each to reduce the particle size of the blank liposomes.
[0065] Encapsulation of weakly basic drugs: the bla...
Embodiment 2
[0067] The preparation of drug-loaded liposomes containing sodium polyaspartate (30mg / mL) in the inner aqueous phase:
[0068] Preparation of blank liposomes: Dissolve distearoylphosphatidylcholine (DSPC), cholesterol, distearoylphosphatidylethanolamine-polyethylene glycol (DSPE-PEG) at a mass ratio of 3:1:0.36 in In water ether, fully dissolve and mix evenly, then evaporate the organic solvent ether under reduced pressure, so that film-forming materials such as phospholipids form a uniform lipid film at the bottom of the flask, and then add 0.25M ammonium sulfate and 30mg / mL sodium polyaspartate (MW=9k) aqueous solution, hydration in 50°C water bath for 30 minutes, vortexed for 5 minutes, using liposome extrusion equipment, extruding the obtained liposome suspension through polycarbonate with a pore size of 200nm and 100nm membranes 10 times each to reduce the particle size of the blank liposomes.
[0069] Encapsulation of weakly basic drugs: the blank liposome obtained in t...
Embodiment 3
[0071] The preparation of drug-loaded liposomes containing sodium polyaspartate (100mg / mL) in the inner aqueous phase:
[0072] Preparation of blank liposomes: Dissolve distearoylphosphatidylcholine (DSPC), cholesterol, distearoylphosphatidylethanolamine-polyethylene glycol (DSPE-PEG) at a mass ratio of 3:1:0.36 in In water ether, fully dissolve and mix evenly, then evaporate the organic solvent ether under reduced pressure, so that the film-forming materials such as phospholipids form a uniform lipid film at the bottom of the flask, and then add 0.25M ammonium sulfate and 100mg / mL sodium polyaspartate (MW=9k) aqueous solution, hydration in 50°C water bath for 30 minutes, vortexed for 5 minutes, using liposome extrusion equipment, extruding the obtained liposome suspension through polycarbonate with a pore size of 200nm and 100nm membranes 10 times each to reduce the particle size of the blank liposomes.
[0073] Encapsulation of weakly basic drugs: the blank liposome obtaine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com