Method, kit and system for verifying blood antibody valence detection capability
A blood group antibody and detection ability technology, applied in the field of blood group antibody titer detection ability verification methods, kits and systems, can solve the problem that cannot be used to evaluate the detection ability of immunohematology laboratories, and the agglutination intensity cannot be truly reflected and used for evaluation. Blood type antibody content and other issues to eliminate obvious damage and improve accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
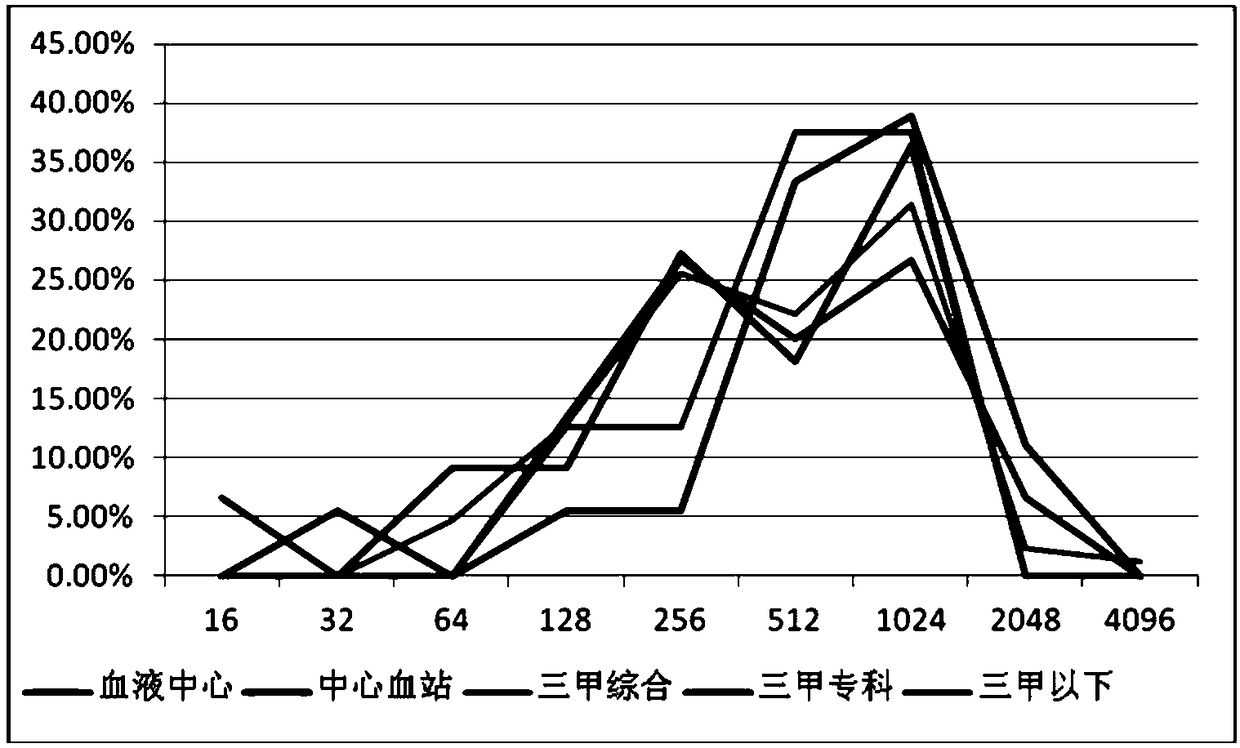
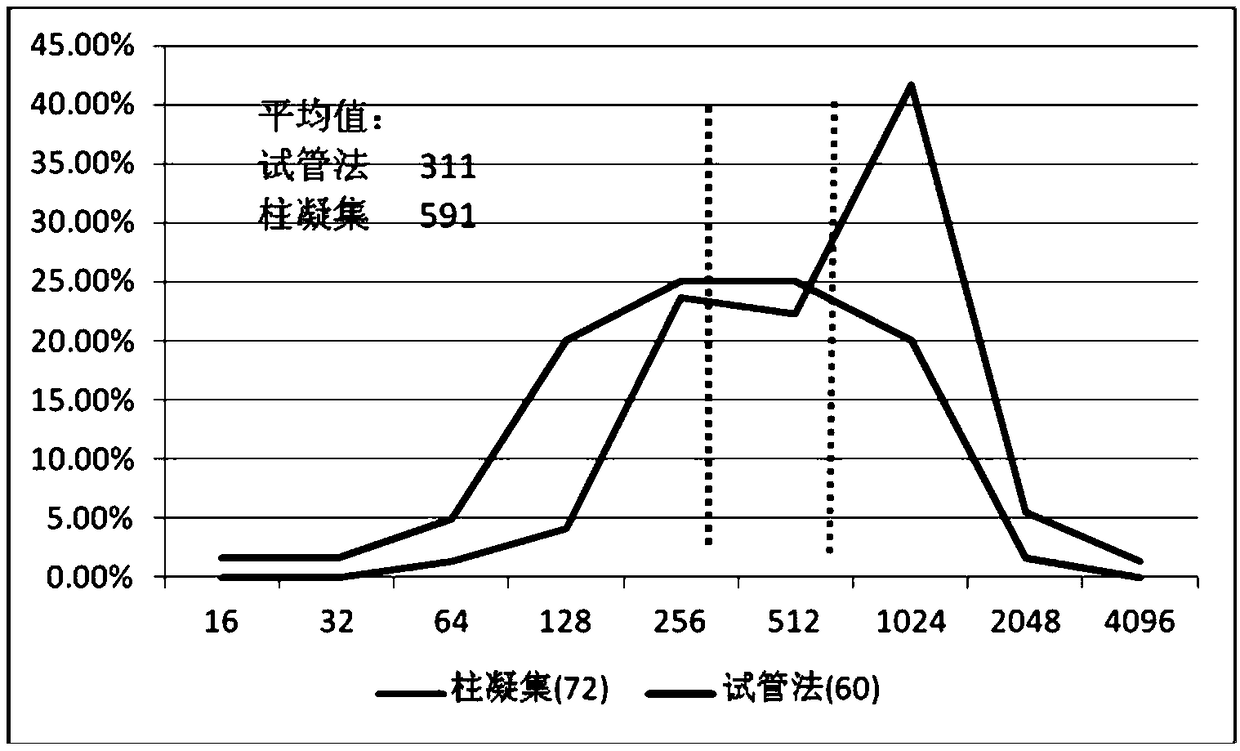
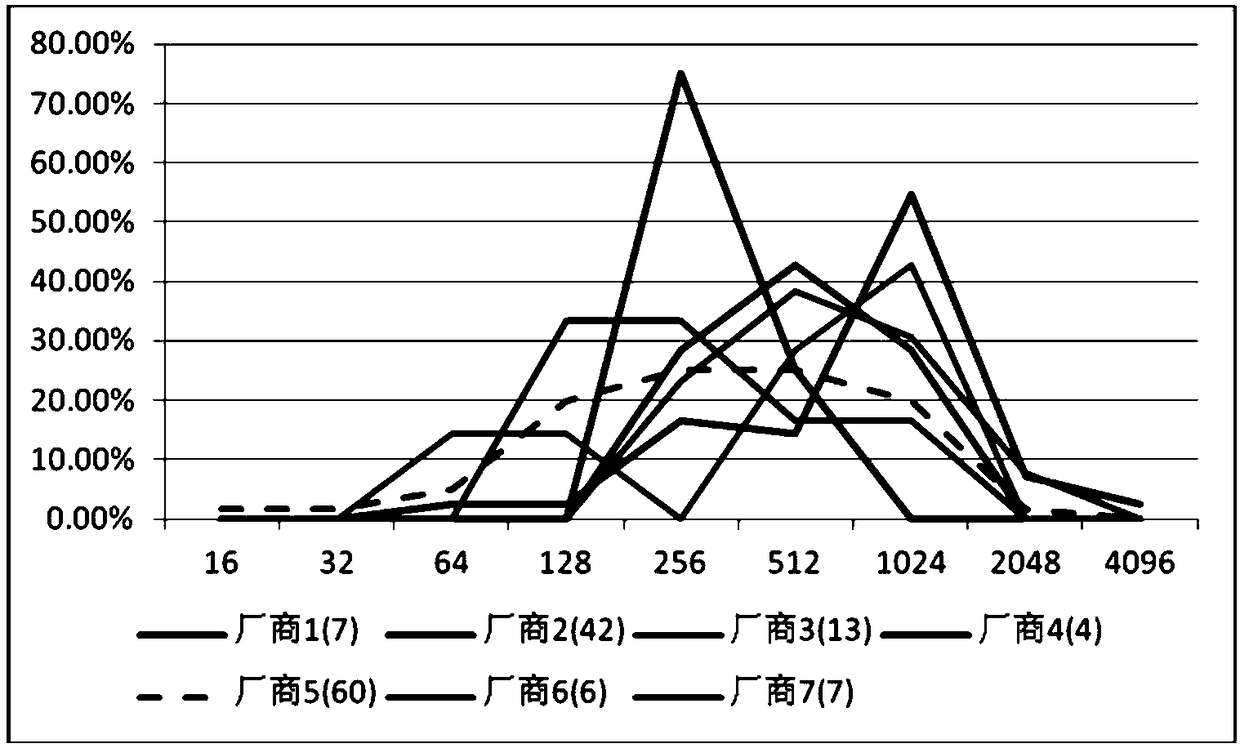
Image
Examples
Embodiment 1
[0051] The nature of blood type antibody can be IgM type and IgG type, and the specificity of blood type antibody can be anti-A, anti-B, anti-D, etc. IgM blood type antibody, because there is no international standard product at present, can only be compared between laboratories and different methods at present. There are international standards for IgG antibodies, which can be compared quantitatively between laboratories. The method is to dilute the raw antibody and the international standard (Bristol International Blood Type Reference Laboratory) by 1.25 times, add the corresponding 2% red blood cell suspension, react and compare in the antiglobulin gel card, and obtain the The precise ratio of the antibody content of the antibody to the international standard. Due to the high titer of the raw antibody, it is usually necessary to quantitatively dilute to a lower concentration before comparing with the standard. Through comparison, the content of raw antibody can be calibra...
Embodiment 2
[0062] see Figure 4 , Figure 4 It is a flow chart of the blood group antibody titer detection ability verification method of the present invention. Include the following steps:
[0063] 1. The evaluator delivers the antibody samples, quality control materials, and instructions to the subjects. The antibody sample is an IgG antibody sample, and its standard titer is known to the evaluator; the quality control product includes a quality control IgG antibody with an agglutination strength of about 2+ (1+ to 3+), and The quality control picture of the quality control IgG antibody reacting with the corresponding erythrocytes at a concentration of 100% to 60%; the instruction manual records the operation method of the quality control product, which is the quality control IgG antibody An operating method that reacts with the corresponding red blood cells, the operating method has specific operating steps and parameters that are consistent with the steps and parameters used by th...
Embodiment 3
[0072] A blood type antibody titer testing proficiency testing kit, comprising:
[0073] Antibody sample: the standard titer of the antibody sample is known to the kit provider;
[0074] Quality control product: the quality control product includes a quality control IgG antibody with an agglutination intensity of about 2+ (1+~3+), and a quality control picture of the quality control IgG antibody reacting with the corresponding red blood cells at a concentration of 100%~60% ;
[0075] Instruction manual: The instruction manual records the operation method of the quality control product. The operation method of the quality control product is the operation method of reacting the quality control IgG antibody with the corresponding red blood cells. The operation method has specific operation steps and parameters, and the operation steps and parameters are the same as those obtained by the kit provider. The steps taken are consistent with the parameters.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com