Method, kit and system for verifying blood serology agglutination intensity judgment capability
A proficiency verification and serological technology, applied in scientific instruments, biological testing, material inspection products, etc., can solve the problems of unseen blood type serological agglutination strength kits and systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
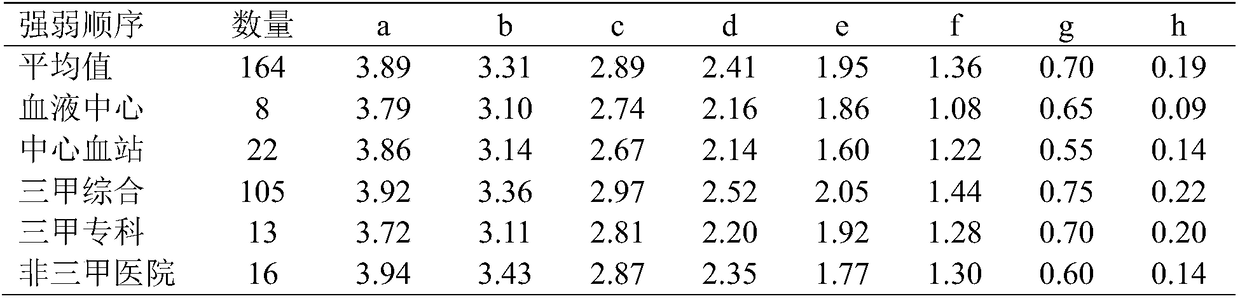
[0047] The method of the present invention for preparing red blood cells with different agglutination intensities utilizes the formation of different quantitative red blood cell antibodies that bind to a certain number of red blood cells to achieve a sequence of strong to weak agglutination strength from 4+ to negative, which is used to verify that the laboratory is relatively The ability to judge the intensity of the reaction. By analyzing the test results of participating laboratories, the ability of the participating laboratories to detect antibodies with different reaction strengths can be accurately evaluated.
[0048] The present invention has been tried out in nearly 200 laboratories in China. From the feedback result analysis, the method can effectively evaluate the laboratory's ability to determine the agglutination intensity, which is of critical significance. Details are as follows:
[0049] 1. Specimen preparation for determination of agglutination strength
[0050] The...
Embodiment 2
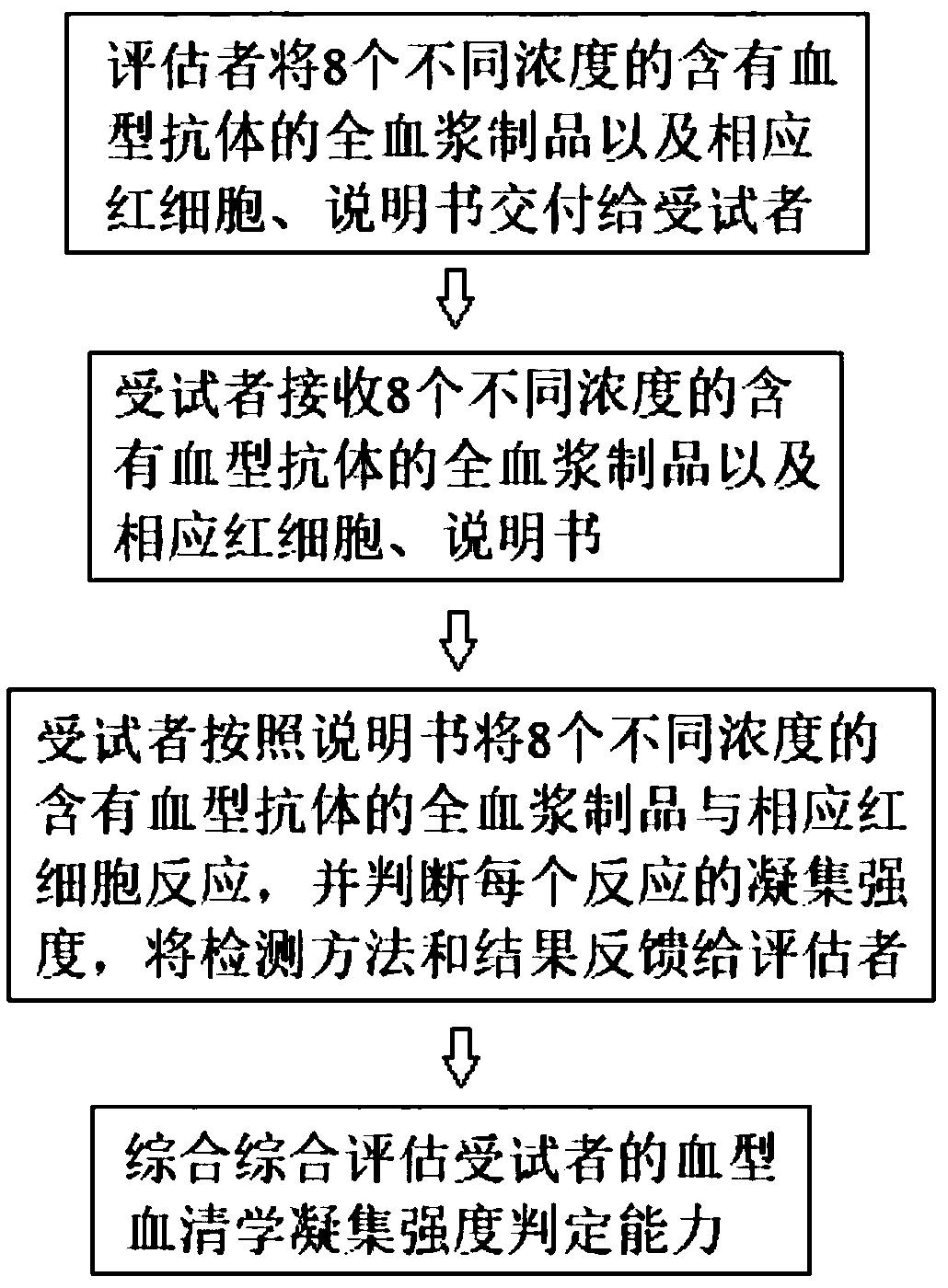
[0064] See figure 1 , figure 1 It is a flowchart of the method for verifying the ability to determine the agglutination strength of blood group serology of the present invention. It includes the following steps:
[0065] 1. The evaluator delivers 8 different concentrations of blood group antibody-containing whole plasma products and corresponding red blood cells and instructions to the subject. The instructions record the requirement to react 8 blood group antibodies with corresponding red blood cells and determine each The aggregation strength of the reaction.
[0066] 2. Subjects receive 8 different concentrations of whole plasma products containing blood group antibodies, as well as corresponding red blood cells and instructions.
[0067] 3. The subject reacted 8 different concentrations of whole plasma products containing blood group antibodies with corresponding red blood cells according to the instructions, and judged the agglutination intensity of each reaction, and fed back ...
Embodiment 3
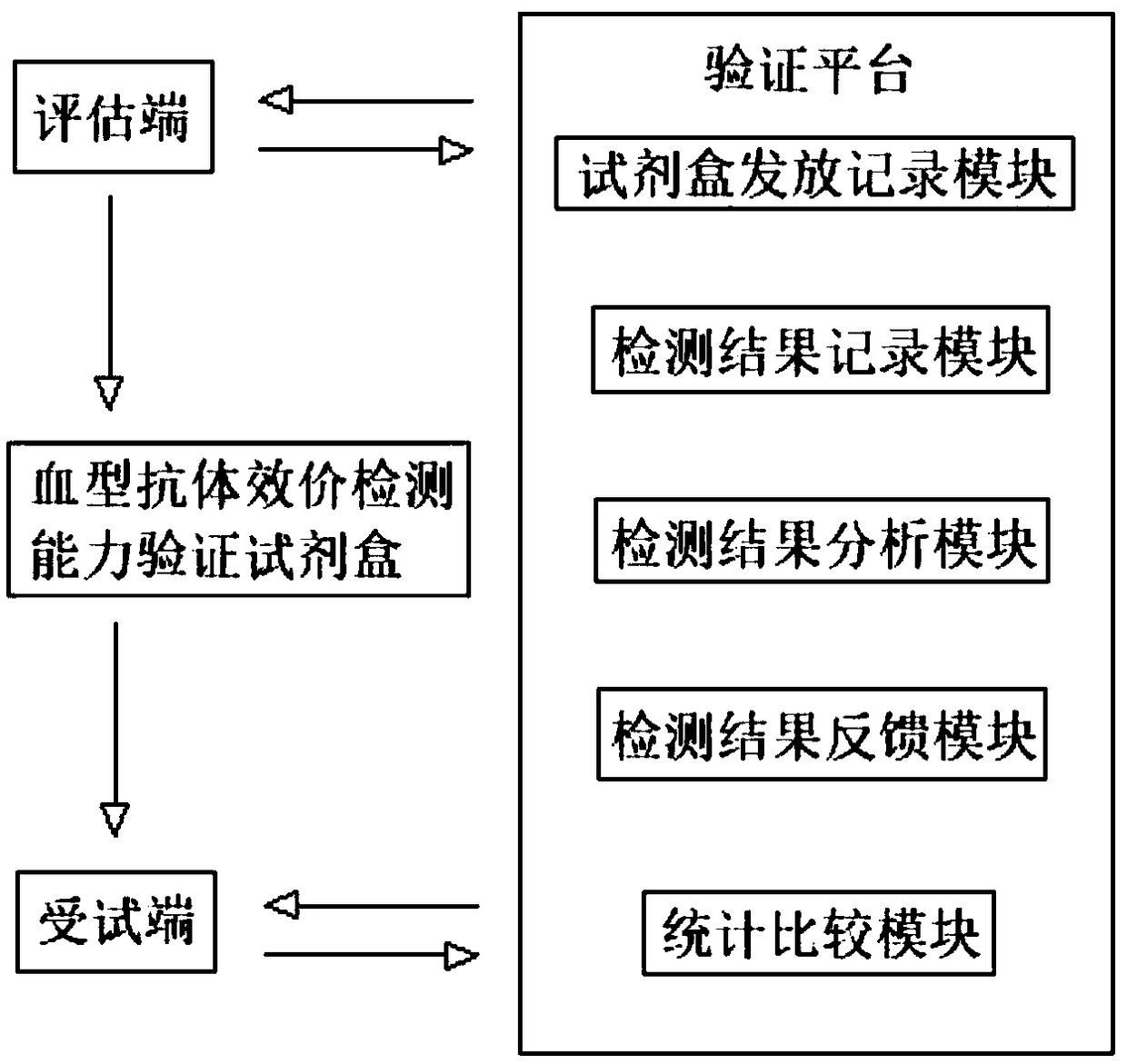
[0071] A blood group serological agglutination strength determination test kit, including:
[0072] 8 different concentrations of whole plasma products containing blood group antibodies;
[0073] Corresponding red blood cells;
[0074] Instructions: Record the need to react 8 different concentrations of whole plasma products containing blood group antibodies with the corresponding red blood cells, and record the detection methods and results.
[0075] Among them, 8 different concentrations of whole plasma products containing blood group antibodies and the corresponding red blood cell reaction have agglutination intensities ranging from 4+ to negative, with the strongest being 4+ and the weakest being negative; and 8 different concentrations of whole blood group antibody-containing whole The agglutination intensity of the plasma product and the corresponding red blood cell reaction is known to the kit provider.
[0076] Wherein, the blood group antibody is selected from any one or more ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com