A kind of synthetic method of 2,5-dibromoiodobenzene
A technology of dibromoiodobenzene and its synthesis method, which is applied in chemical instruments and methods, preparation of amino compounds, preparation of organic compounds, etc., can solve the problems of raw material monopoly, monopoly, unfavorable scale and popularization of industrial production, and achieve The effect of large quantity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
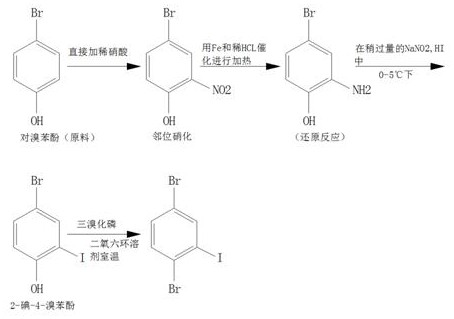
[0021] A kind of synthetic method of 2,5-dibromoiodobenzene that the present invention proposes, comprises the following steps: S1, the synthesis of 2-nitro-4-bromophenol: add p-bromophenol in chemical reaction still, then under room temperature condition Add dilute nitric acid directly under the pressure, let it stand for 20min, carry out fractional distillation of the reactant under reduced pressure, collect 125°C, 2.35kPa fraction to obtain 2-nitro-4-bromophenol;
[0022] S2, the synthesis of 2-amino-4-bromophenol: put 2-nitro-4-bromophenol in a chemical reaction kettle, add Fe and dilute HCl to 2-nitro-4-bromophenol, and carry out Heating to realize ortho-position nitration, keeping the temperature at 70°C for 1 hour, fractionating the reactant under reduced pressure, collecting fractions at 105°C and 2.57kPa to obtain 2-amino-4-bromophenol;
[0023] Synthesis of S3, 2-iodo-4-bromophenol: add a little excess NaNO to the reaction kettle 2 , HI, then add 2-amino-4-bromophen...
Embodiment 2
[0026] A kind of synthetic method of 2,5-dibromoiodobenzene that the present invention proposes, comprises the following steps: S1, the synthesis of 2-nitro-4-bromophenol: add p-bromophenol in chemical reaction still, then under room temperature condition Add dilute nitric acid directly under the pressure, let it stand for 30min, carry out fractional distillation of the reactant under reduced pressure, collect 125°C, 2.35kPa fraction to obtain 2-nitro-4-bromophenol;
[0027] S2, the synthesis of 2-amino-4-bromophenol: put 2-nitro-4-bromophenol in a chemical reaction kettle, add Fe and dilute HCl to 2-nitro-4-bromophenol, and carry out Heating to realize ortho nitration, the temperature is kept at 75°C for 1.5h, the reactant is fractionated under reduced pressure, and the fraction at 110°C and 2.63kPa is collected to obtain 2-amino-4-bromophenol;
[0028] Synthesis of S3, 2-iodo-4-bromophenol: add a little excess NaNO to the reaction kettle 2 , HI, then add 2-amino-4-bromophen...
Embodiment 3
[0031] A kind of synthetic method of 2,5-dibromoiodobenzene that the present invention proposes, comprises the following steps: S1, the synthesis of 2-nitro-4-bromophenol: add p-bromophenol in chemical reaction still, then under room temperature condition Add dilute nitric acid directly under the pressure, let it stand for 35min, carry out fractional distillation under reduced pressure, collect 130°C, 2.95kPa distillate to obtain 2-nitro-4-bromophenol;
[0032] S2, the synthesis of 2-amino-4-bromophenol: put 2-nitro-4-bromophenol in a chemical reaction kettle, add Fe and dilute HCl to 2-nitro-4-bromophenol, and carry out Heating to realize ortho nitration, keeping the temperature at 80°C for 1.5h, fractionating the reactant under reduced pressure, collecting fractions at 120°C and 2.73kPa to obtain 2-amino-4-bromophenol;
[0033] Synthesis of S3, 2-iodo-4-bromophenol: add a little excess NaNO to the reaction kettle 2 , HI, then add 2-amino-4-bromophenol, keep the temperature ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com