Integral membrane protein display on poxvirus extracellular enveloped virions
A technology that integrates membrane proteins and poxviruses, and is used in viruses, viruses/phages, fusion polypeptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0039] Furthermore, "and / or" as used herein should be construed as specifically disclosing each of the two features or components, with or without the other. Thus, the term "and / or" as used herein in the phrase "A and / or B" is intended to include "A and B", "A or B", "A" (alone), and "B" (alone). Likewise, the term "and / or" used in phrases such as "A, B, and / or C" is intended to include the various embodiments of: A, B, and C; A, B, or C; A or C; A or B; B or C; A and C; A and B; B and C; A (alone); B (alone); and C (alone).
[0040] Unless defined otherwise, technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this disclosure belongs. For example, "Concise Dictionary of Biomedicine and Molecular Biology" (Concise Dictionary of Biomedicine and Molecular Biology), Juo, Pei-Show, 2nd edition, 2002, CRC Press (CRCPress); "Dictionary of Cell and Molecular Biology" (The Dictionary of Cell and Molecula...
Embodiment 1
[0207] Example 1: Fusion protein construction
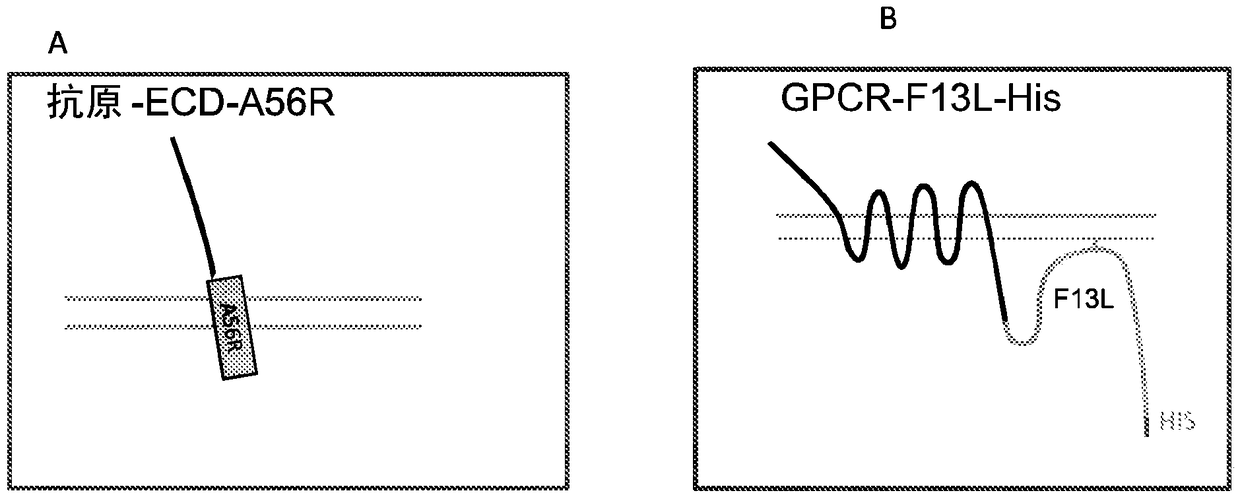
[0208] The IMP was introduced into the vaccinia virus EEV by using the EEV specific proteins F13L, A56R and B5R as follows. In general, the extracellular domains of HER2, CD100 (axon guidance factor 4D) and FZD4 were introduced as fusions with single-transmembrane EEV-specific membrane proteins A56R and B5R, as schematically shown in Figure 1A. The mature FZD4-ECD-A56R fusion protein comprises amino acids 20-370 of SEQ ID NO:6, the mature HER2-ECD-A56R fusion protein comprises amino acids 20-855 of SEQ ID NO:7, and the mature CD100-ECD-A56R fusion protein comprises SEQ ID NO:7 Amino acids 20-935 of ID NO:8. Figure 1B and Figure 1C It is schematically shown how multi-transmembrane proteins such as GPCR and CD20 can be introduced into EEV as multi-transmembrane proteins in the form of fusions with the EEV membrane-associated protein F13L.
[0209] Preparation of F13L fusion proteins (FZD4-F13L, CD20-F13L and CXCR4-F13L)
[0210...
Embodiment 2
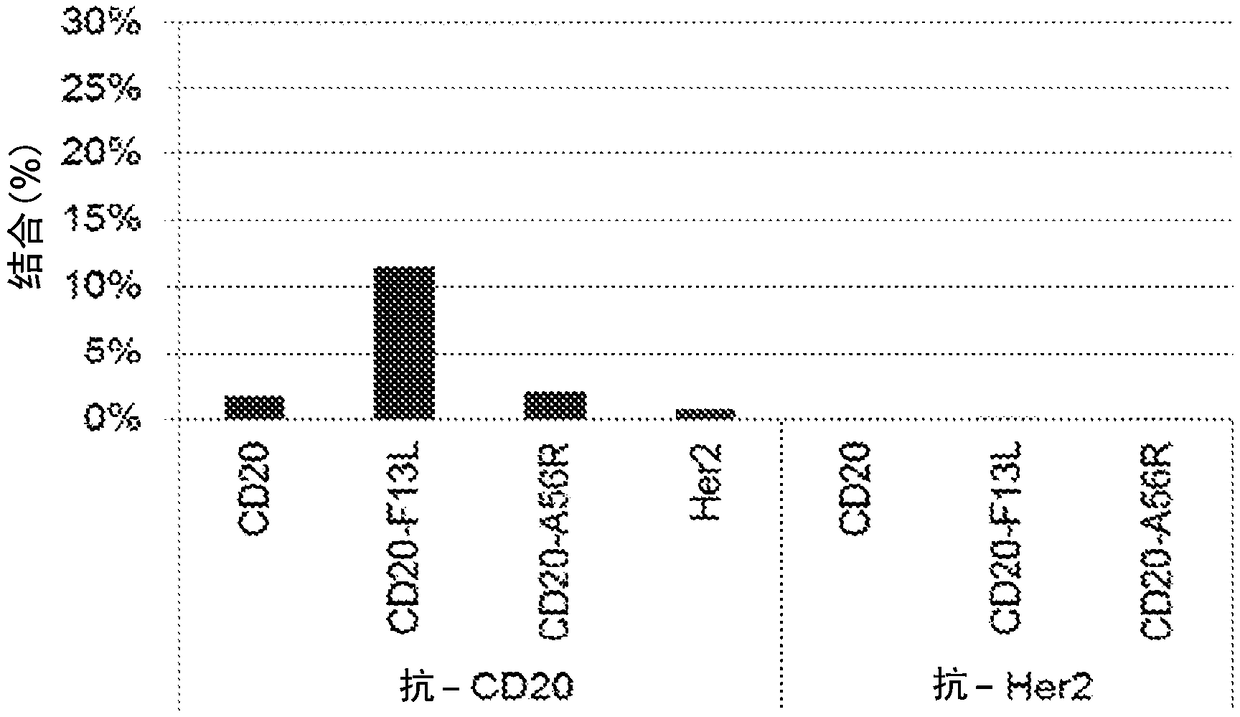
[0220] Example 2: Expression of CD20-F13L fusion protein on EEV
[0221] BHK cells were infected with IMV encoding CD20-F13L fusion protein (SEQ ID NO:4) or control Western Reserve (WR) virus at a multiplicity of infection (MOI) of 1 virus / cell for two days, and then EEV-containing cells were harvested by low-speed centrifugation. supernatant and remove debris. Protein G (110 μL) was pulled down with a magnet, and 1 mL of PBS + 20 μg of purified anti-CD20 antibody was added to the beads. The solution was incubated at room temperature for 30-60 minutes with gentle shaking to allow antibody coupling to the protein G beads. 10 μg of purified mIgG1 isotype control was added to the solution to ensure complete blocking, and the solution was then incubated for another 10-30 minutes at room temperature with gentle shaking. The beads were pulled down with a magnet, washed once with 1 mL of PBS, and resuspended in 110 μL of PBS.
[0222] 50 μL of anti-CD20-Pro G Add to 1 mL of CD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com