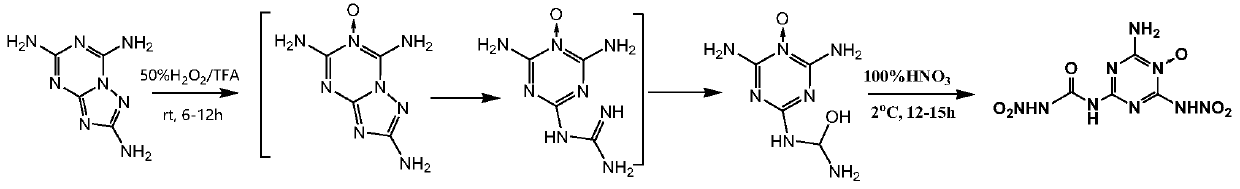
2-Amino-4-nitroamine-6-formylnitroamine-1,3,5-triazine nitrogen oxide and its preparation method
A formyl nitramide and nitrogen oxide technology, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of low triazine ring tension and difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
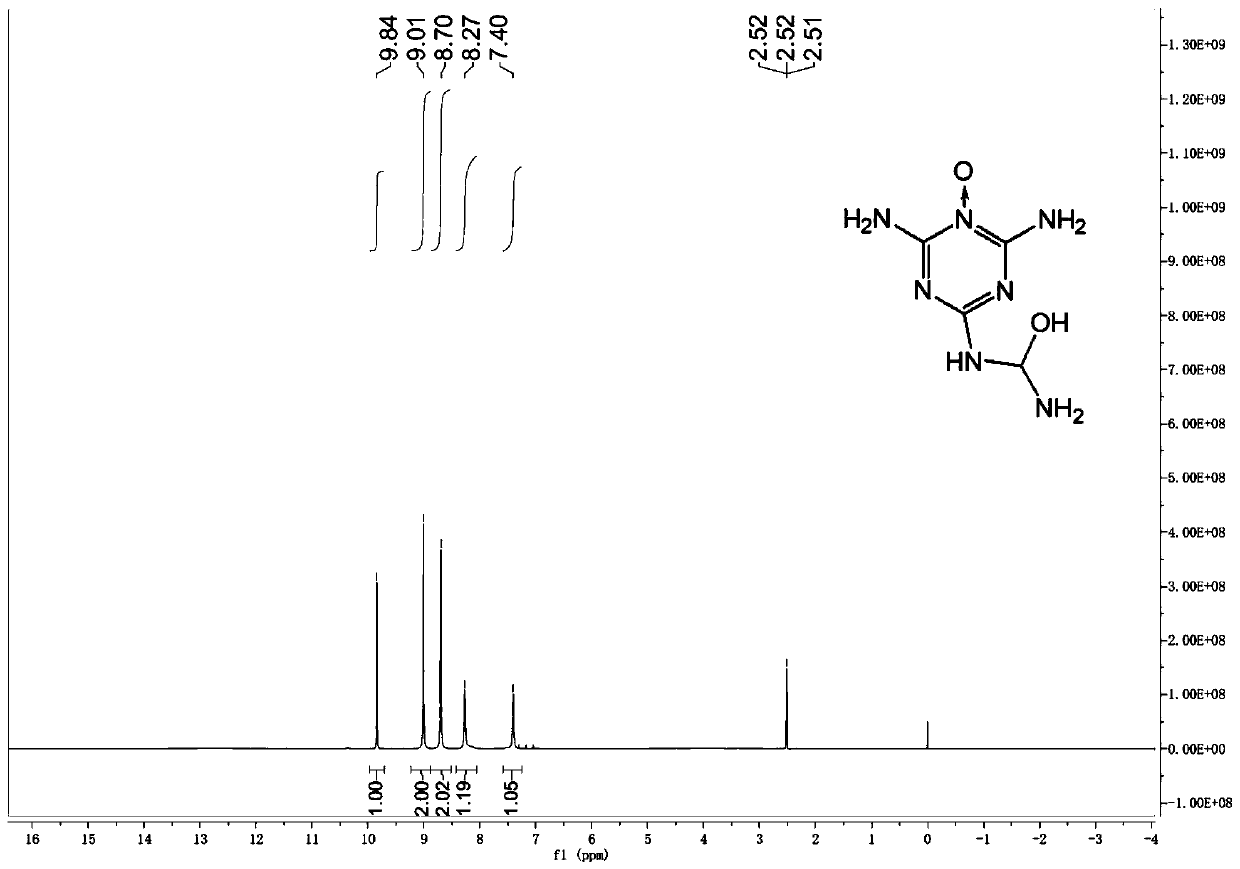
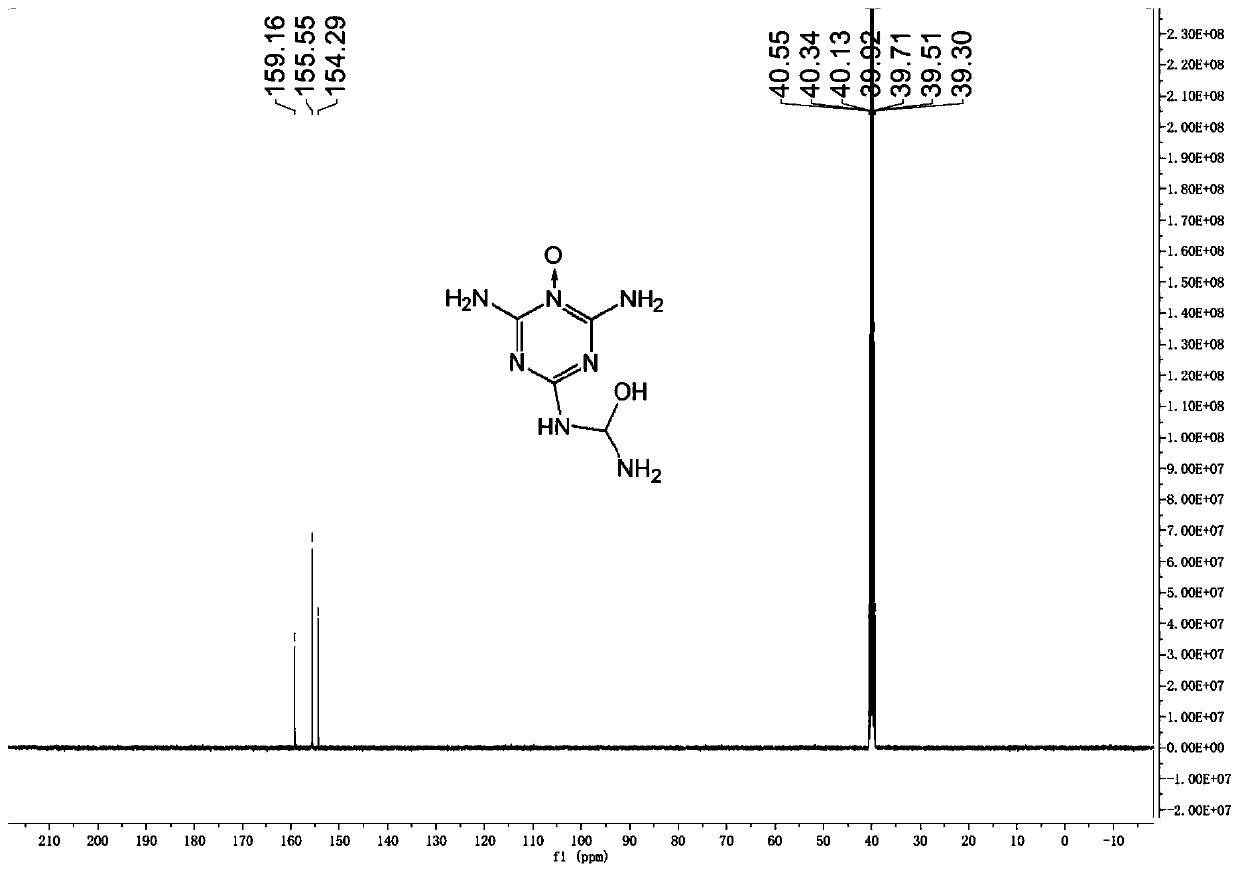
[0025] Under magnetic stirring at 25°C, dissolve 0.83g (5mmol) of 1,3,5-triazino1,2,4-triazole in 10mL (0.135mol) of trifluoroacetic acid, and slowly drop 5mL (0.166mol) of 50% hydrogen peroxide, the feeding temperature should not exceed 40°C. After dropping, reacted at 25°C for 5h, poured into ice water to quench, filtered out the solid, washed with water, and dried to obtain 0.39g of intermediate 1,3,5-triazine nitrogen oxide derivative, with a yield of 42%. At -5°C, dissolve 0.19 g (1 mmol) of the above intermediate in 2 mL of 100% HNO 3 (48mmol), returned to 2°C to continue the reaction for 2h, then poured into ice water to quench, stirred for 0.5h, filtered out the precipitate, washed the filter cake with water, and dried in vacuo to obtain white powder 2-amino-4-nitroamine-6- Formyl nitramide-1,3,5-triazine nitrogen oxide 53 mg, yield 19%.
Embodiment 2
[0027] Under magnetic stirring at 25°C, dissolve 0.83g (5mmol) of 1,3,5-triazino1,2,4-triazole in 20mL (0.269mol) of trifluoroacetic acid, and slowly drop 5mL (0.166mol) of 50% hydrogen peroxide, the feeding temperature should not exceed 40°C. After dropping, react at 25° C. for 5 h, pour into ice water to quench, filter out the solid, wash with water, and dry to obtain 0.28 g of the intermediate 1,3,5-triazine nitrogen oxide derivative, with a yield of 30%. At -5°C, dissolve 0.19 g (1 mmol) of the above intermediate in 2 mL of 100% HNO 3 (48mmol), returned to 2°C to continue the reaction for 2h, then poured into ice water to quench, stirred for 0.5h, filtered out the precipitate, washed the filter cake with water, and dried in vacuo to obtain white powder 2-amino-4-nitroamine-6- Formyl nitramide-1,3,5-triazine nitrogen oxide 53 mg, yield 19%.
Embodiment 3
[0029] Under magnetic stirring at 25°C, dissolve 0.83g (5mmol) of 1,3,5-triazino1,2,4-triazole in 15mL (0.202mol) of trifluoroacetic acid, and slowly drop 8mL (0.266mol) 50% hydrogen peroxide, the feeding temperature should not exceed 40°C. After dropping, reacted at 25°C for 5h, poured into ice water to quench, filtered out the solid, washed with water, and dried to obtain 0.49g of the intermediate 1,3,5-triazine nitrogen oxide derivative, with a yield of 52%. At -5°C, dissolve 0.57 g (3 mmol) of the above intermediate in 8 mL of 100% HNO 3 (0.144mol), return to 2°C and continue to react for 2h, then pour into ice water to quench, stir for 0.5h, filter out the precipitate, wash the filter cake with water, and vacuum dry to obtain white powder 2-amino-4-nitroamine-6 - 0.16 g of formyl nitramide-1,3,5-triazine nitrogen oxide, yield 19%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| impact sensitivity | aaaaa | aaaaa |
| impact sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com