Preparation method of key intermediate of paroxetine hydrochloride
A technology of paroxetine hydrochloride and intermediates, which is applied in the field of drug synthesis, can solve problems such as environmental pollution and waste, and achieve the effects of improving enterprise benefits and reducing input costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
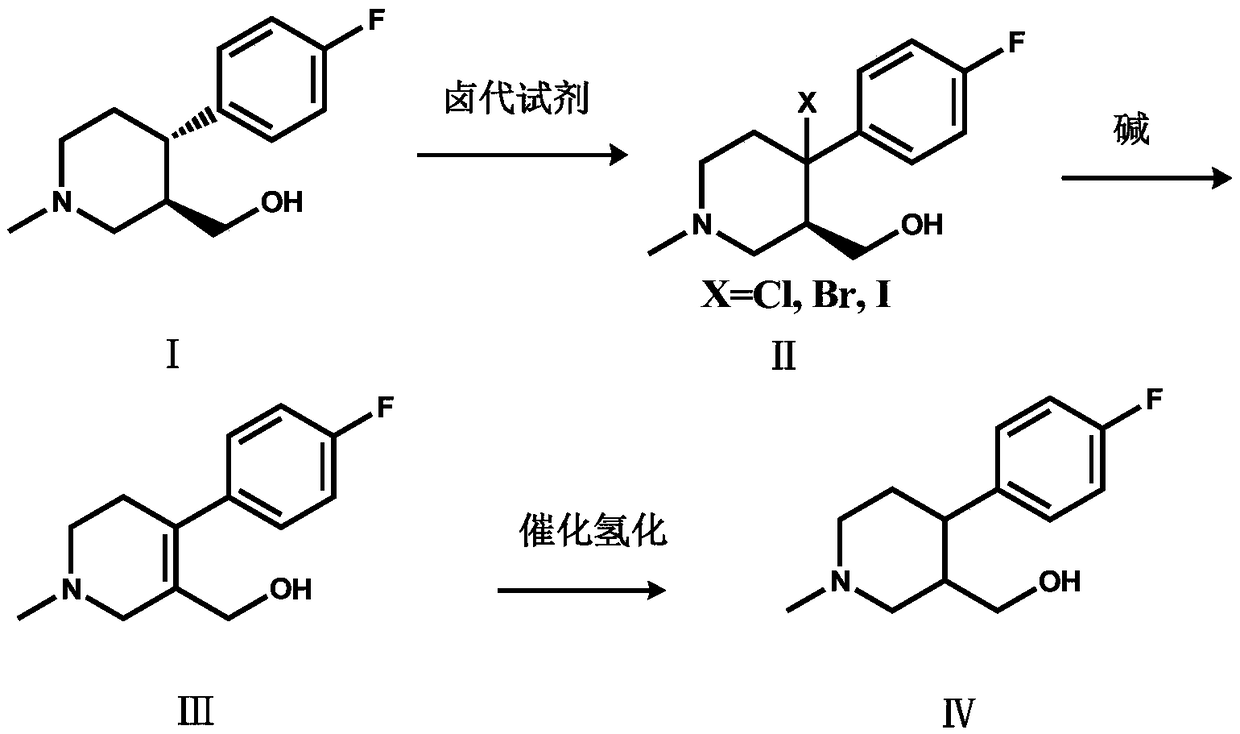
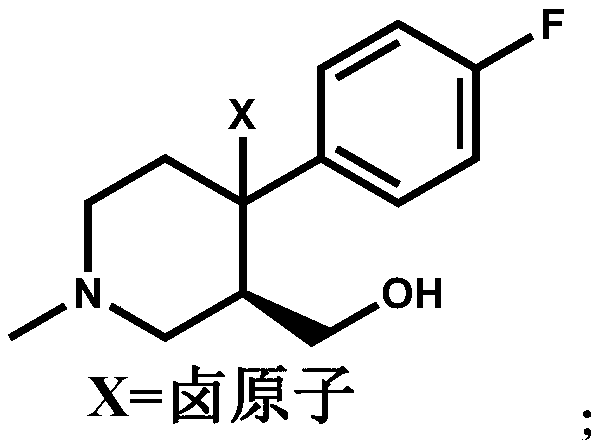
[0021] Add (+)-trans-4-(4-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine (15.00g, 0.0672mol) and BPO (0.488g, 0.0020mol) into a 250ml three-necked flask ), add 80ml of dichloromethane to dissolve compound I, add NBS (17.94g, 0.1008mol) dichloromethane solution (concentration: 0.5mol / L) dropwise at room temperature, continue to stir for 6 hours after the addition is completed, and the HPLC central control shows the reaction End, then dropwise add 30ml of water to quench the reaction, separate the layers, extract the aqueous layer three times with dichloromethane (40ml×3 times), combine the organic layer, wash the organic layer three times with water (30ml×3 times), dry over anhydrous sodium sulfate, iso Compound II (16.84 g, 0.05575 mol) was obtained by beating with propanol, and the molar yield was 82.96%.
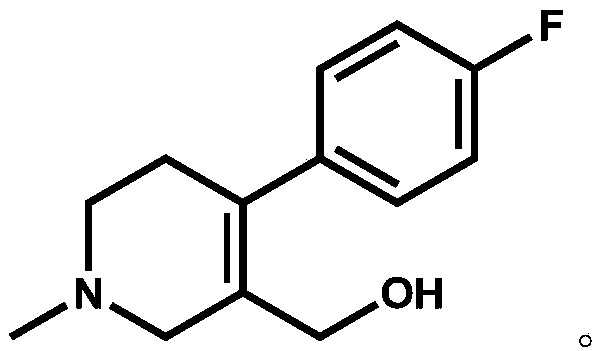
[0022] Add compound II (X=Br, 15.00g, 0.0496mol) and 90ml 1,2-dichloroethane into a 250ml three-necked flask, raise the temperature, and add 60ml N,N-diisopropylethylami...
Embodiment 2
[0025] Add (+)-trans-4-(4-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine (15.00g, 0.0672mol) and azobisisobutyronitrile ( 0.328g, 0.0020mol), add 80ml tetrahydrofuran to dissolve compound Ⅰ, add NCS (17.95g, 0.1344mol) tetrahydrofuran solution (concentration: 0.7mol / L) dropwise at room temperature, continue stirring for 6 hours after the addition is completed, and the HPLC central control displays After the reaction was completed, 30ml of water was added dropwise to quench the reaction, concentrated to dry tetrahydrofuran, and separated, the aqueous layer was extracted three times with dichloromethane (40ml×3 times), the organic layer was combined, and the organic layer was washed three times with water (30ml×3 times). Drying over sodium sulfate and beating with isopropanol gave Compound II (13.07 g, 0.0507 mol), with a molar yield of 75.50%.
[0026] Add compound II (X=Cl, 12.78g, 0.0496mol) and 90ml toluene to a 250ml three-necked flask, raise the temperature, and add 60ml...
Embodiment 3
[0029]Add (+)-trans-4-(4-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine (15.00g, 0.0672mol) and BPO (0.488g, 0.0020mol) into a 250ml three-necked flask ), add 80ml DMF to dissolve compound I, add NIS (27.22g, 0.121mol) DMF solution (concentration: 0.8mol / L) dropwise at room temperature, continue to stir for 6 hours after the addition is completed, the HPLC central control shows that the reaction is over, and then drop Add 30ml of water to quench the reaction, separate the layers, extract the aqueous layer three times with ethyl acetate (40ml×3 times), combine the organic layer, wash the organic layer three times with water (30ml×3 times), dry over anhydrous sodium sulfate, and beat with MTBE to obtain compound II (18.61g, 0.05329mol), molar yield 79.30%.
[0030] Add compound II (X=I, 17.32g, 0.0496mol) and 90ml chloroform into a 250ml three-necked flask, heat up, and add 60ml N,N-diisopropylethylamine (16.03g, 0.124mol) dropwise in chloroform at 60°C (Concentration is 1mol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com