Anti-human PD1 monoclonal antibody and application thereof
A monoclonal antibody, antibody technology, applied in the direction of antibody, anti-animal/human immunoglobulin, application, etc., can solve the problems of lack of MYPPY, inability to bind B7-1 and B7-2, etc., to achieve the effect of novel sequence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Obtaining specific anti-PD1 mouse monoclonal antibody by fusion hybridoma technology
[0040] 1.1 Animal immunity
[0041] Mice were immunized according to the general method in the literature (E Harlow, D. Lane, Antibody: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., 1998). The immunogen was recombinant human PD1 (amino acid range Leu 25-Gln 167) protein (Acrobiosystems, Cat#PD1-H5257) containing a human IgG1 Fc tag at the C-terminus. Recombinant human PD1 protein with his s tag (Sino biological inc., cat#10377-H08H) was used as the detection antigen for serum titer determination and hybridoma screening. Briefly, remove an appropriate amount of Freund's adjuvant into a 1.5ml EP tube, and shake to mix. Prepare the antigenic protein solution with PBS. Mix the adjuvant and protein antigen solution according to the required amount, fully emulsify the antigen by pushing each other through the syringe to form a stable water-...
Embodiment 2
[0047] Example 2 In vitro analysis method for determining the functional activity of PD1 monoclonal antibody
[0048] 2.1 Determination of antibody binding ability based on capture ELSIA
[0049] Prepare goat anti-mouse IgG Fcr-specific secondary antibody (Jackson Immuno Research, #115-006-071) in 1xPBS to a final concentration of 2 μg / ml, add 100 μl / well to a 96-well microtiter plate, 4 degrees Pack overnight. The next day, after washing the plate 4 times with PBS solution containing 0.05% Tween 20 (ie 1xPBST), 200 μl / well of 5% skim milk powder in PBST solution was added and placed at 37°C for 2 hours to block. Wash the plate again, add 100 μl / well of diluted antibody solution or hybridoma supernatant, incubate at 37 degrees for 40 minutes, and then wash the plate 4 times. Add the prepared 60nM biotin-labeled human PD1-Fc protein solution (in 2.5% skimmed milk powder in PBST) at 100 μl / well, incubate at 37°C for 40 minutes, and then wash the plate 4 times. Add 1:10000 dil...
Embodiment 3
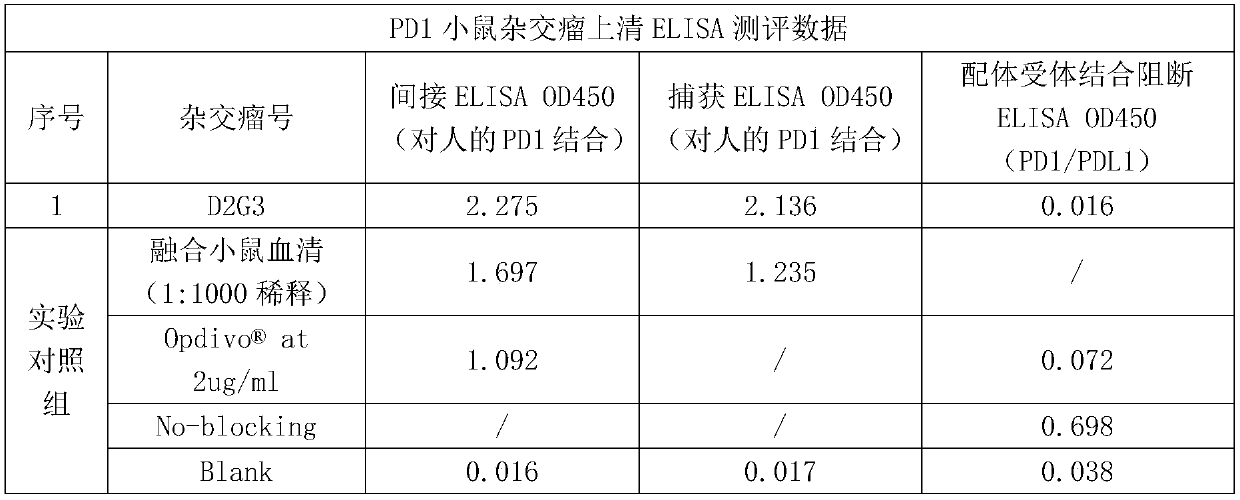
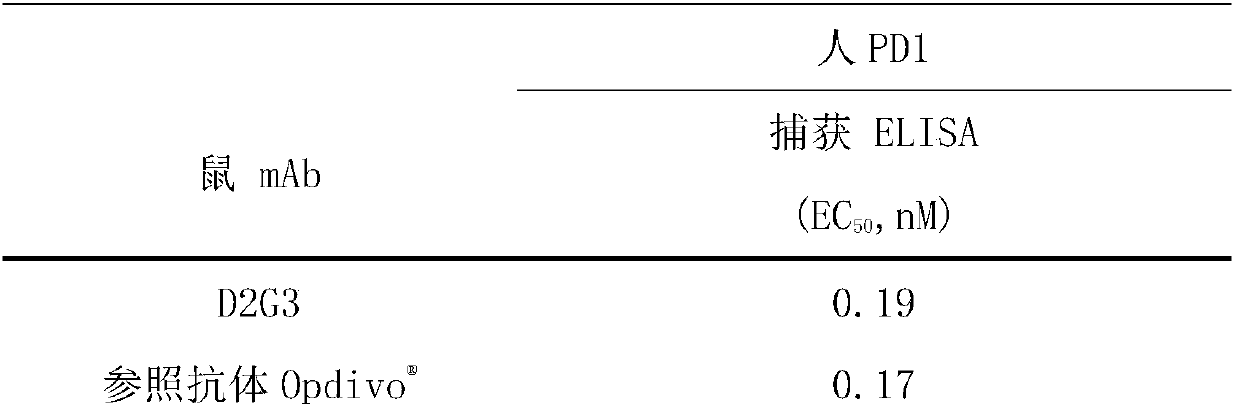
[0059] Example 3 Binding activity of anti-PD1 mouse monoclonal antibody
[0060] According to the analysis method described in Example 2.1, the evaluation of the binding activity of the PD1 mouse monoclonal antibody is summarized in Table 2 below. where the reference antibody As a control, an existing commercialized anti-human PD1 monoclonal antibody was used. It can be seen from Table 2 that the binding activity of the anti-human PD1 monoclonal antibody of the present invention to human PD1 antigen is equivalent to that of the reference antibody.
[0061] Table 2 Binding activity of PD1 antibody
[0062]
[0063]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com