Application of SAG in preparation of drug for treating diseases of hypoxic-ischemic brain damage during development
A technology for hypoxia-ischemia and developmental stage, which is applied in the fields of cardiovascular system diseases, drug combinations, and pharmaceutical formulas. It can solve the problems of incompletely clear pathogenesis of HIE, limited treatment methods, lack of effective treatment methods, etc., and achieve obvious social benefits. and economical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] 1. Experimental materials and reagents
[0019] 1.1. Experimental animals
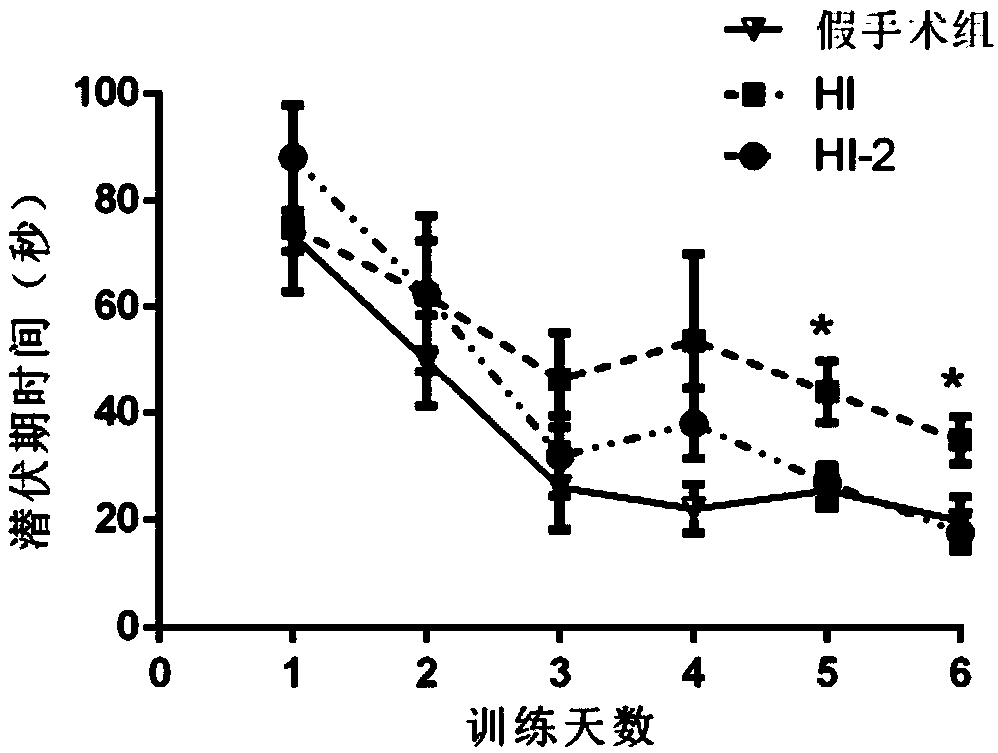
[0020] Healthy and clean 7-day-old newborn SD rats, male or female, weighing 14-22 grams, were purchased from Chengdu Dashuo Experimental Animal Co., Ltd. The experiment is divided into two parts, the first part is to determine the appropriate SAG concentration. Randomly divided into sham operation group, solvent control group, SAG high-dose experimental group (25mg / kg), SAG medium-dose experimental group (15mg / kg), SAG low-dose experimental group (5mg / kg), each group of 12 SD large mouse. Solvent control group, SAG high-dose experimental group, SAG medium-dose experimental group, and SAG low-dose experimental group were intraperitoneally injected with solvent (double distilled water) or corresponding concentration of SAG 2 hours after modeling. According to the results of TTC and H&E staining, the suitable dose group of SAG was determined. The second part will be randomly divided into sham ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com