Improved methods for reprograming non-pluripotent cells into pluripotent stem cells
A reprogramming and cell technology, applied in artificially induced pluripotent cells, embryonic cells, gastrointestinal cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0238] Example 1: Chemical surrogates for Oct4
[0239] To identify chemical surrogates for Oct4, MEFs from OG mice were seeded at a density of 20,000 cells / well of a 12-well plate and infected with lentiviruses encoding Sox2, Klf4 and c-Myc. After infection, the medium was replaced with LIF-free ESC medium. Individual chemicals from the small molecule library are added to each well. Media and chemicals were changed every 4 days. Chemical treatment was continued for 14-20 days or until GFP positive colonies appeared. The primary hit was selected for further confirmation and optimization.
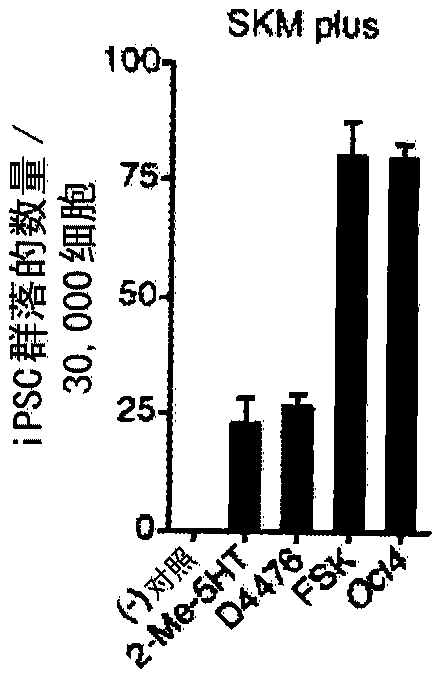
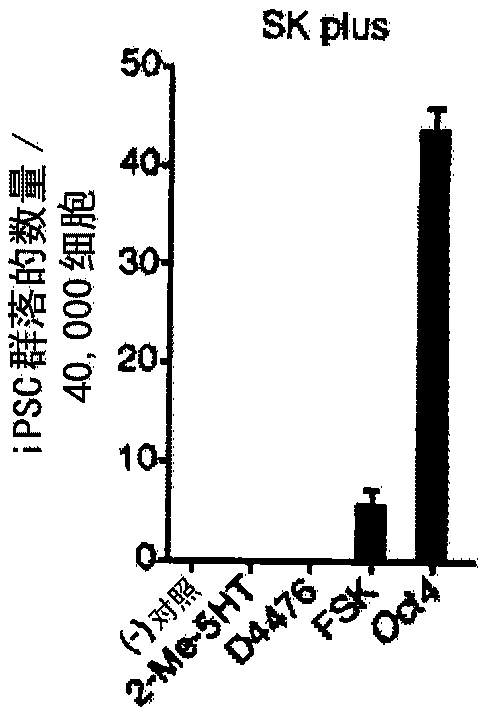
[0240] In the presence of viral expression of Sox2, Klf4, and c-Myc, the use of Oct4 promoter-driven green fluorescent protein (GFP) expression (OG) mouse embryonic fibroblast (MEF) searches enabled Small molecules for reprogramming. After screening up to 10,000 small molecules (Table 1C), forskolin (FSK), 2-methyl-5-hydroxytryptamine (2-Me-5HT), and D4476 (Table 1D) were identified as ...
Embodiment 2
[0243] Example 2: Small Molecules that Promote Late Reprogramming
[0244] To identify small molecules that promote late reprogramming, a doxycycline (DOX)-induced Oct4 expression screening system was used. (Li et al., Cell Res. 21:196-204 (2011)).
[0245] MEFs from OG mice were inoculated as described above and infected with Fu-tet-hOct4 and FUdeltaGW-rtTA lentiviruses. The induction protocol was performed as described above.
[0246] After infection, the medium was replaced with LIF-free ESC medium containing VC6T (VPA, CHIR99021, 616452, tranylcypromine) plus DOX (1 μg / ml). Alternatively, B6 from Tet-On POU5F1 mouse strain; 129-Gt(ROSA)26Sor tm1(rtTA*M2)Jae Collal tm2(tetO-Pou5f1)Jae / J MEFs carrying DOX-inducible Oct4. (Li et al., Cell Res., 21:196-204 (2011)). These two DOX-inducible systems were used only in this screen and not in full chemical reprogramming. Individual chemicals from the small molecule library are added to each well. Concentrations of small mol...
Embodiment 3
[0248] Example 3: Complete chemical reprogramming without the use of Oct-4 inducible systems
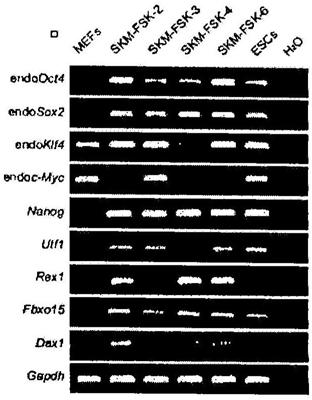
[0249] To achieve complete chemical reprogramming without using an Oct4-inducible system, small molecules were further tested in chemical reprogramming of OG-MEFs without transgenes. When DZNep was added 16 days after treatment with VC6TF (VC6TFZ), GFP-positive cells were obtained with up to 65 times more frequency than those treated with VC6TF, forming dense, epithelial, GFP positive community ( Figure 4A -B and 4E). In these cells, the expression levels of most pluripotency marker genes were increased, but still lower than in ESCs, indicating an incomplete reprogramming state (Fig. 4G and H). Certain GFP-positive colonies formed ESC-like morphology (dome , translucent, uniform, with clear edges)( Figure 4C ) (Silva et al., PLoS Biol., 6:e253 (2008); Theunissen et al., Curr. Biol., 21:65-71 (2011)). These colonies can be further cultured for more than 30 generations, maintain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com