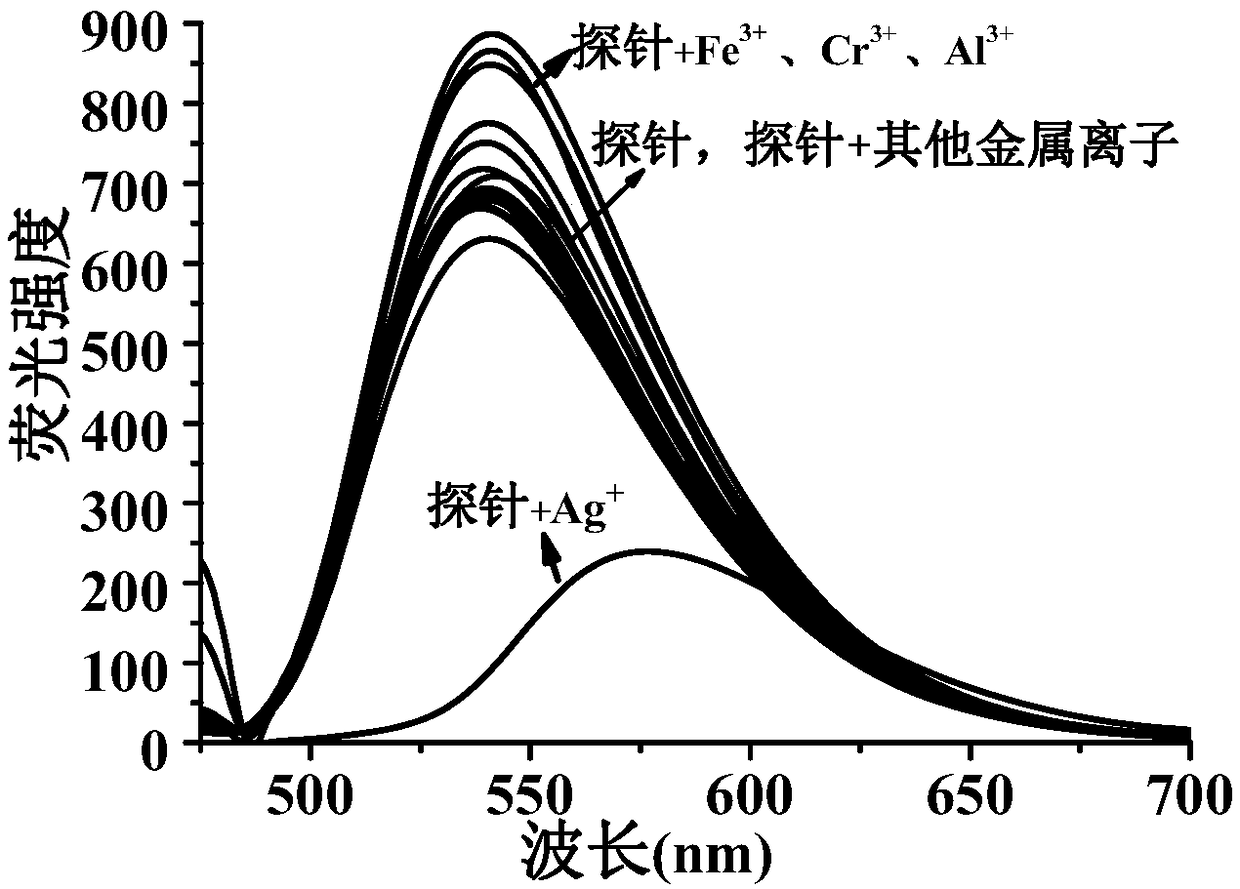
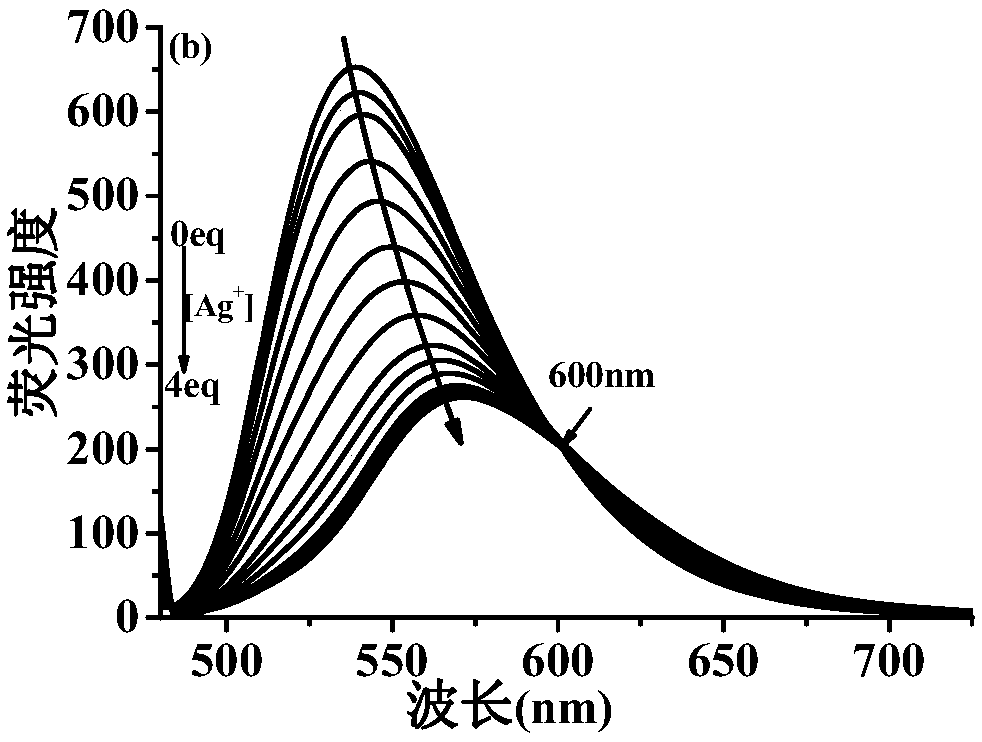
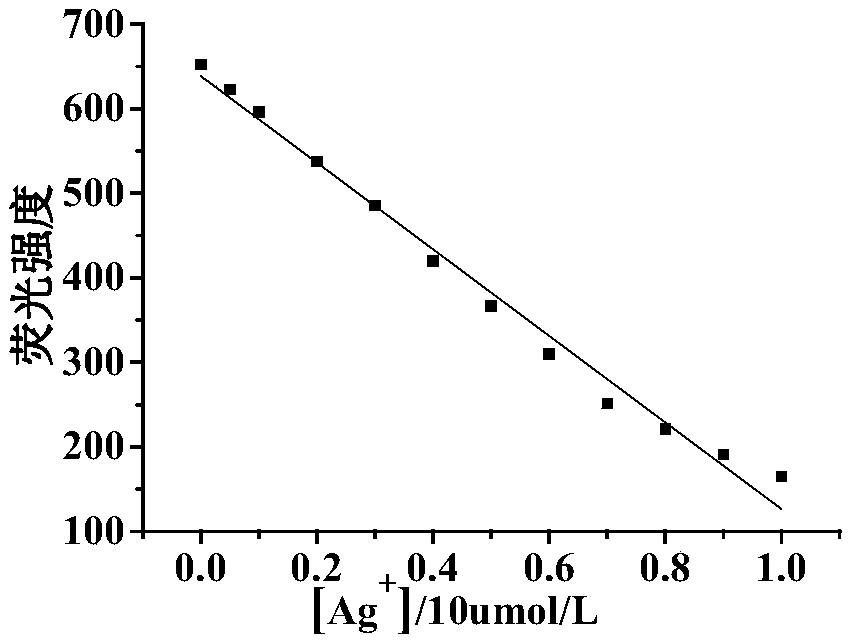
Ag+ fluorescent probe and Ag+ chemical sensor
A fluorescent probe and sensor technology, applied in the field of fluorescent probes, to achieve the effects of colorimetric detection with naked eyes, reversible and non-destructive fluorescence, and bright colors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] Correspondingly, another aspect of the embodiment of the present invention provides an Ag + The preparation method of fluorescent probe comprises the following steps:
[0043] S01. Under the protection of nitrogen or inert gas, add compound 1 shown in the following structure, anhydrous acetone, and cesium carbonate to a dry reaction bottle, and perform the first heating and reflux treatment; after cooling, add compound 2 and iodide shown in the following structure Cesium is heated and refluxed for the second time to prepare compound 3 shown in the following structure, and the reaction formula is as follows:
[0044]
[0045] S02. Under the protection of nitrogen or inert gas, add compound 3, absolute ethanol, and hydrazine hydrate to a dry reaction bottle, heat and reflux for the third time, filter out the white insoluble solid after the reaction, and evaporate the filtrate to dryness with chloroform methanol Recrystallize to prepare compound 4 shown in the followin...
Embodiment 1
[0071] a kind of Ag + The preparation method of fluorescent probe (hereinafter referred to as probe), comprises the following steps:
[0072] S11. Synthesis of compound 3
[0073] Under nitrogen protection, compound 1 (500mg, 0.48mmol) and 100mL of dry acetone were added to a dry 250ml three-neck flask, stirred to dissolve, cesium carbonate (1.56g, 4.8mmol) was added, and heated to reflux for 1h. After it was cooled, compound 2 (264mg, 1.2mmol) and cesium iodide (124mg, 0.48mmol) were added, and heated to reflux for 6h. After the reaction was completed, the solvent was evaporated to dryness, extracted with chloroform (3×100 mL), the organic phase was washed with 200 mL of saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. Chloroform / methanol (100 / :2) was separated and purified by silica gel column chromatography to obtain 0.46 g of yellow solid compound 3 with a yield of 67.3%.
[0074] The NMR data of compound 3 ...
Embodiment 2
[0088] The preparation method of various reagents in the analytical method of the present invention:
[0089] (1) Preparation of probe stock solution: Weigh 75.1 mg of the probe reagent prepared in Example 1, dissolve it with N,N-dimethylformamide and acetonitrile respectively, and prepare Ag +Fluorescent probe concentration of 1mM N,N-dimethylformamide solution (N,N-dimethylformamide probe stock solution) 50mL, Ag + Fluorescent probe concentration of 1mM acetonitrile solution (acetonitrile probe stock solution) 50mL.
[0090] (2)Ag + Preparation of stock solution: Weigh 90.4 mg of silver perchlorate monohydrate, dissolve it in ultrapure water, and prepare 10 mL of a solution with a concentration of 20 mM.
[0091] (3) Other metal ions Li + , Na + , K + , Mg 2+ , Ca 2+ , Ba 2+- , Hg 2+ ,Sr 2+ , Zn 2+ , Al 3+ , Fe 3+ ,Co 2+ , Ni 2+ , Cu 2+ , Zn 2+ , Pb 2+ , Cd 2+ , Mn 2+ Preparation of the stock solution: Take the perchlorate of the corresponding metal ion r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com