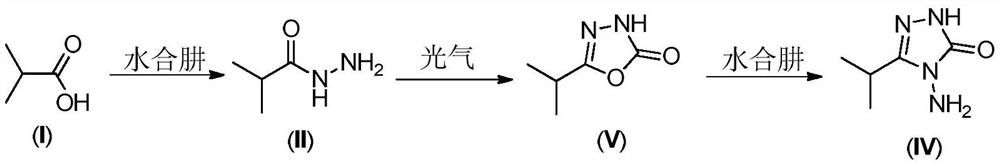
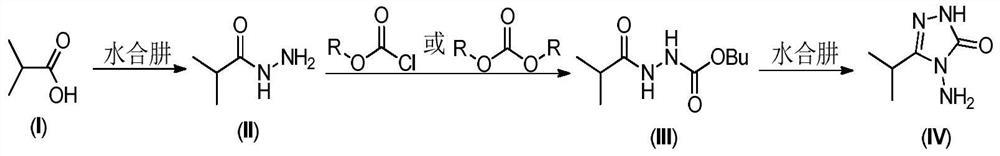
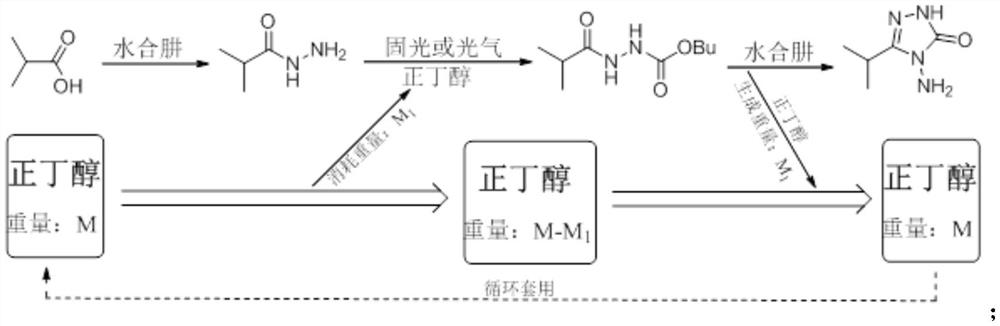
A method for preparing 3-isopropyl-4-amino-1,2,4-triazolin-5-one
A technology of triazoline and isopropyl, which is applied in the field of pesticide synthesis, can solve the problems of low recovery rate, difficult separation, and increased equipment cost, and achieve the effects of reducing separation operations and equipment, simplifying the reaction system, and fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] At room temperature, 44.5g (0.50mol) of isobutyric acid and 222.5g of n-butanol were put into a 500mL reaction flask, stirring was started, and 32.9g (0.525mol) of 80% hydrazine hydrate was slowly added dropwise. After the dropwise addition was completed, the temperature was raised to reflux for dehydration. When 23mL of water came out, the heating was turned off, and the temperature of the water bath was lowered to 15-20°C to obtain product 1. 50.0 g (0.50 mol) of phosgene was slowly introduced at 15-20° C. for 1 hour, and kept at this temperature for 3 hours to obtain product 2. Afterwards, 31.3g (0.50mol) of 80% hydrazine hydrate and 8.0g (0.1mol) of 50% sodium hydroxide were added, and the temperature was raised to 105-110° C. for reflux for 3 hours, and the reaction was completed. Stand for stratification, and separate the n-butanol in the upper layer. Continue to cool the lower water phase to 20-40°C, adjust the pH to 6-7 with 30% hydrochloric acid, then continu...
Embodiment 2
[0031] At room temperature, put 44.5g (0.50mol) of isobutyric acid and 133.5g of n-butanol into a 500mL reaction flask, start stirring, and slowly add 46.9g (0.75mol) of 80% hydrazine hydrate dropwise. After the dropwise addition was completed, the temperature was raised to reflux for dehydration. When 35mL of water was removed, the heating was turned off, and the temperature of the water bath was lowered to 15-20°C to obtain product 1. Slowly add 55.0 g (0.183 mol) of solid light at 15-20° C. for 1 hour, and keep the temperature for 3 hours to obtain product 2. Add 31.3g (0.50mol) 80% hydrazine hydrate and 24.0g (0.3mol) 50% sodium hydroxide afterwards, heat up to 100-105° C. and reflux for 3 hours, and the reaction ends. Stand for stratification, and separate the n-butanol in the upper layer. Continue to cool the lower water phase to 20-40°C, adjust the pH to 6-7 with 30% hydrochloric acid, then continue to cool down to 0-5°C, and continue to stir at this temperature for h...
Embodiment 3
[0034] At room temperature, 44.5g (0.50mol) of isobutyric acid and 133.5g of n-butanol were put into a 500mL reaction flask, and stirring was started, while 32.9g (0.525mol) of 80% hydrazine hydrate was slowly added dropwise. After the dropwise addition was completed, the temperature was raised to reflux for dehydration. When 23mL of water came out, the heating was turned off, and the temperature of the water bath was lowered to 10-15°C to obtain product 1. 52.5 g (0.525 mol) of phosgene was slowly introduced at 10-15° C. for 2 hours, and kept at this temperature for 3 hours to obtain product 2. Afterwards, 31.3g (0.50mol) of 80% hydrazine hydrate and 10.7g (0.1mol) of sodium carbonate were added, and the temperature was raised to 95-100° C. for reflux for 3 hours, and the reaction was completed. Stand for stratification, and separate the n-butanol in the upper layer. Continue to cool the lower water phase to 20-40°C, adjust the pH to 6-7 with 30% hydrochloric acid, then con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com