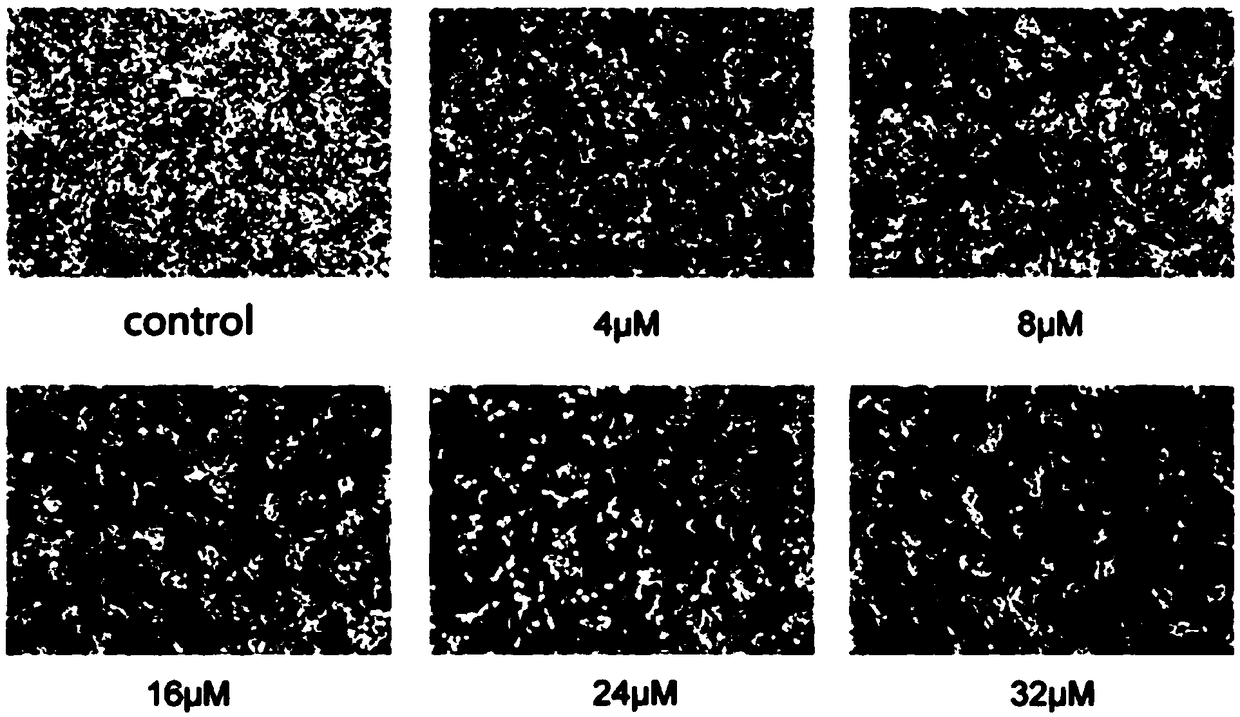
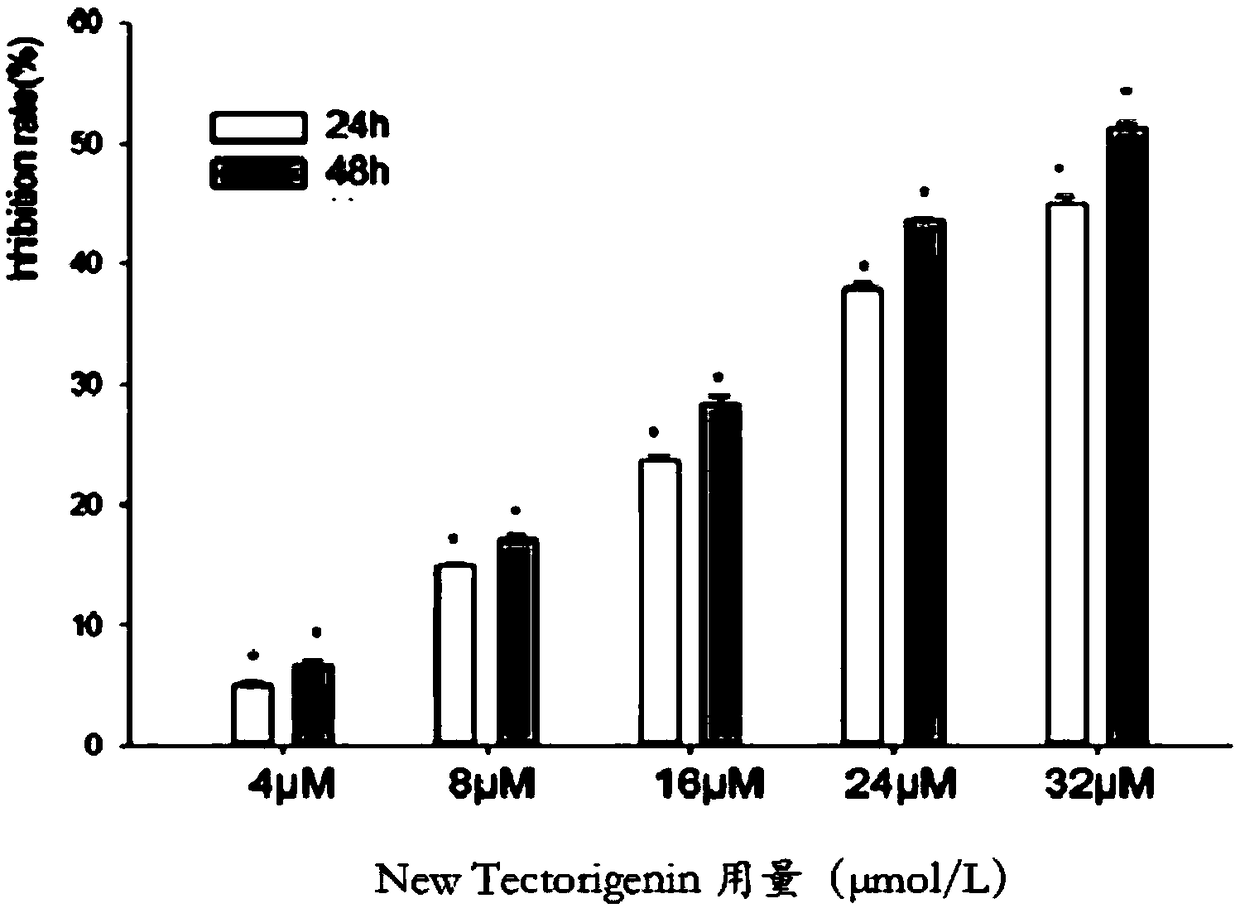
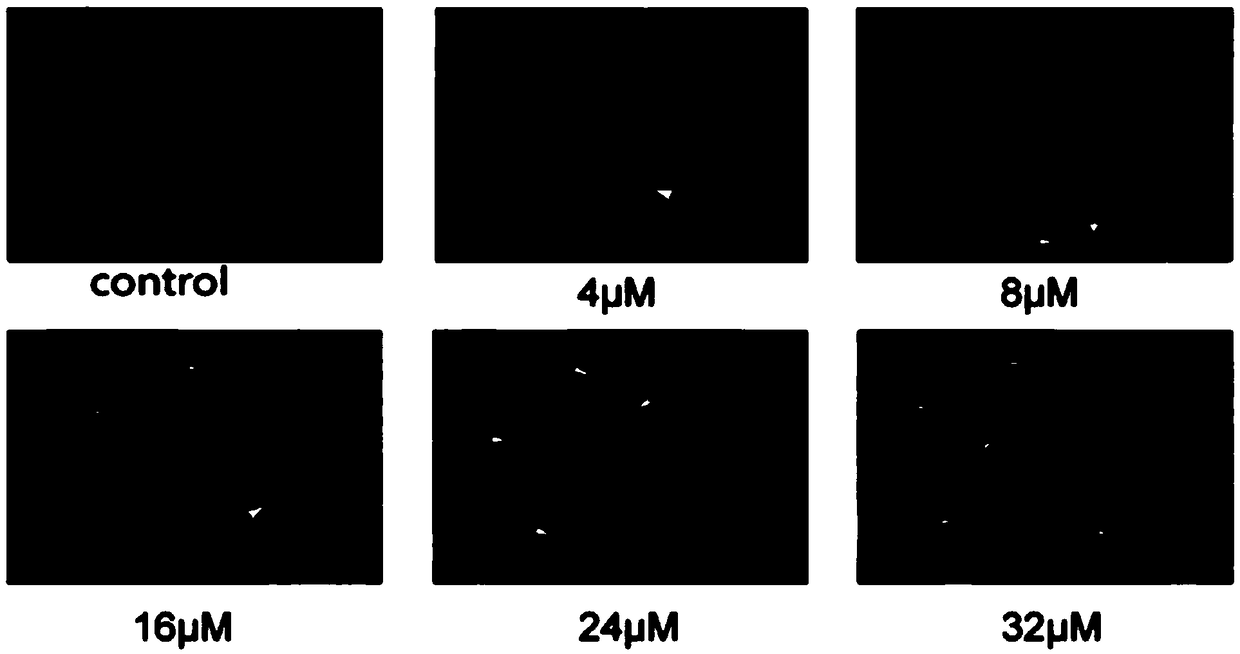
Application of quinoline derivatives of N-isostere tectorigenin in anti-hepatocarcinoma drugs
A technology of isosteric and irisin, applied in the application field of quinoline derivatives in anti-cancer drugs, to achieve the effect of improving ROS generation, significant therapeutic effect and inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] A kind of embodiment of the preparation method of the quinoline derivative of N electron isostere irisin of the present invention, comprises the steps:
[0063] (1) Dissolve 1,3-dibromo-2-methoxy-5-nitrobenzene in 10 equivalents of ethanol at room temperature; add 6 equivalents of 10% Na to the resulting solution 2 S 2 o 4 aqueous solution, stirred at 50°C for 7 hours; when the reaction mixture was cooled to room temperature, extracted three times with AcOEt (ethyl acetate), each time with 2-4 times the volume; the total extract was washed with saturated saline solution and washed with MgSO 4 Drying; AcOEt was removed under reduced pressure to obtain 3,5-dibromo-4-methoxyaniline as a white powder;
[0064] (2) Dissolve 3,5-dibromo-4-methoxyaniline in 10 equivalents of CH(OMe) at room temperature 3 , stirred for 6-12 hours; excess CH(OMe) was removed 3 Afterwards, (cis)-N-3,5-dibromo-4-methoxyphenylcarboximide was obtained as a white powder;
[0065] (3) Dissolve (c...
Embodiment 2
[0071] A kind of embodiment of the preparation method of the quinoline derivative of N electron isostere irisin of the present invention, comprises the steps:
[0072] (1) Dissolve 1,3-dibromo-2-methoxy-5-nitrobenzene in 15 equivalents of ethanol at room temperature; add 2 equivalents of 20% Na to the resulting solution 2 S 2 o 4 aqueous solution, stirred at 55°C for 5 hours; when the reaction mixture was cooled to room temperature, extracted three times with AcOEt, each time with 2-4 times the volume; the total extract was washed with saturated saline solution and washed with MgSO 4 Drying; AcOEt was removed under reduced pressure to obtain 3,5-dibromo-4-methoxyaniline as a white powder;
[0073] (2) Dissolve 3,5-dibromo-4-methoxyaniline in 16 equivalents of CH(OMe) at room temperature 3 , stirred for 6-12 hours; excess CH(OMe) was removed 3 Afterwards, (cis)-N-3,5-dibromo-4-methoxyphenylcarboximide was obtained as a white powder;
[0074] (3) Dissolve (cis)-N-3,5-dibrom...
Embodiment 3
[0078] A kind of embodiment of the preparation method of the quinoline derivative of N electron isostere irisin of the present invention, comprises the steps:
[0079] (1) Dissolve 1,3-dibromo-2-methoxy-5-nitrobenzene in 20 equivalents of ethanol at room temperature; add 4 equivalents of 30% Na to the resulting solution 2 S 2 o 4 aqueous solution, stirred at 60°C for 3 hours; when the reaction mixture was cooled to room temperature, extracted three times with AcOEt, each time with 2-4 times the volume; the total extract was washed with saturated saline solution and washed with MgSO 4 Drying; AcOEt was removed under reduced pressure to obtain 3,5-dibromo-4-methoxyaniline as a white powder;
[0080] (2) Dissolve 3,5-dibromo-4-methoxyaniline in 20 equivalents of CH(OMe) at room temperature 3 , stirred for 6-12 hours; excess CH(OMe) was removed 3 Afterwards, (cis)-N-3,5-dibromo-4-methoxyphenylcarboximide was obtained as a white powder;
[0081] (3) Dissolve (cis)-N-3,5-dibrom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com