A method and application of increasing monoclonal antibody production
A monoclonal antibody and yield technology, applied in chemical instruments and methods, biochemical equipment and methods, peptides, etc., can solve problems such as high cost and complicated process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The screening process of parameter in the process of embodiment 1 present invention
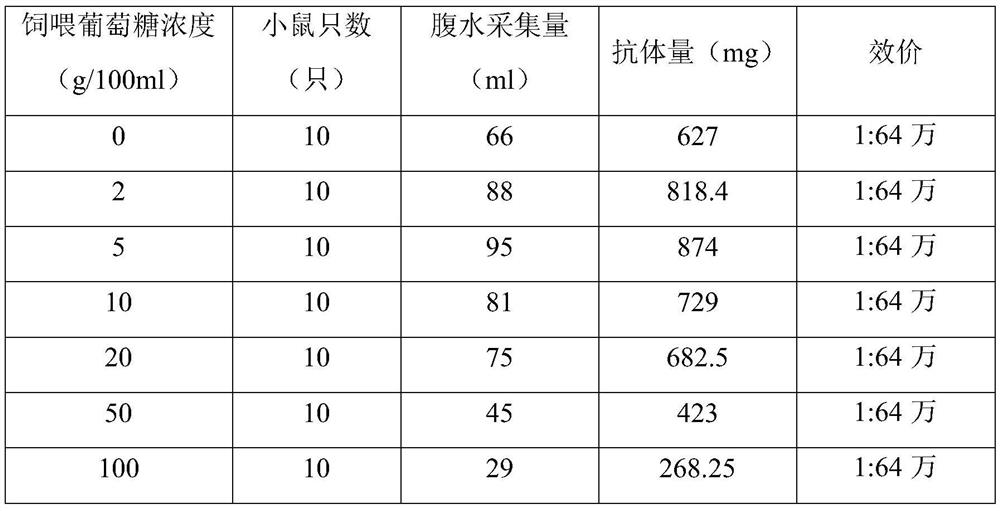
[0044] 1. The screening of feeding glucose concentration is as follows:
[0045] (1) The anti-human NT-proBNP 6C7 hybridoma cells were cloned and cultured, and expanded in cell culture flasks. 10-14 days before the hybridoma cells were injected, 0.5ml / mouse of liquid paraffin was injected intraperitoneally into the mouse, and one week later, Dilute to 0.9×10 with PBS for injection 6 The hybridoma cells / ml were injected into the abdominal cavity of mice, 0.5ml / only, and a total of 70 mice were injected, divided into 7 groups;
[0046] (2) Feed the mice with pure water as the water source first, inject the cultured anti-human NT-proBNP 6C7 hybridoma cells into the abdominal cavity of the mice, and after 5 days, feed the mice in the 7 groups with a concentration of 0 %, 2%, 5%, 10%, 20%, 50%, 100% glucose solution, fed twice a day, fed with glucose until the antibody was collected;
[...
Embodiment 2
[0089] Embodiment 2 A preparation method of anti-human NT-proBNP monoclonal antibody 6C7, comprising the steps of:
[0090] (1) The anti-human NT-proBNP 6C7 hybridoma cells were cloned and cultured, and after being identified as all positive by ELISA, they were expanded in cell culture flasks, and one week before the intraperitoneal injection of the hybridoma cells, 0.5ml / head of liquid paraffin was injected for one week After injection, dilute to 0.9×10 with PBS 6 Hybridoma cells / ml were injected into the peritoneal cavity of mice, 0.5ml / only, and a total of 10 mice were injected;
[0091] (2) The mice were fed pure water all the time, and after the cultured anti-human NT-proBNP 6C7 hybridoma cells were injected into the abdominal cavity of the mice for 5 days, the mice were fed with 5% glucose, twice a day, and fed with Until the antibody collection is completed;
[0092] (3) 7-14 days after the cultured anti-human NT-proBNP 6C7 hybridoma cells were injected into the mice,...
Embodiment 3
[0098] The screening process of parameter in the process of embodiment 3 present invention
[0099] 1. The screening of feeding glucose concentration is as follows:
[0100] (1) Cloning and culturing the anti-human D-dimer 1D10 hybridoma cells, and expanding the culture in cell culture flasks. 10-14 days before injecting the hybridoma cells, intraperitoneally inject 0.5ml / mouse of liquid paraffin into the mouse, and one week later , diluted to 0.9×10 with PBS for injection 6 The hybridoma cells / ml were injected into the abdominal cavity of mice, 0.5ml / only, and a total of 70 mice were injected, divided into 7 groups;
[0101] (2) The mice were first fed with pure water as a water source, and the cultured anti-human D-dimer 1D10 hybridoma cells were injected into the abdominal cavity of the mice. After 5 days, the mice in the 7 groups were fed with a concentration of 0%, 2%, 5%, 10%, 20%, 50%, 100% glucose solution, fed once a day, fed with glucose until the antibody was coll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com