Real-time fluorescent quantitative PCR primer pair and detection kit for detecting hepatospora eriocheir and application of detection kit
A real-time fluorescence quantitative, detection kit technology, applied in the determination/inspection of microorganisms, resistance to vector-borne diseases, biochemical equipment and methods, etc. Problems such as false positives occur, and the results are intuitive, the detection efficiency is improved, and the sensitivity is high.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
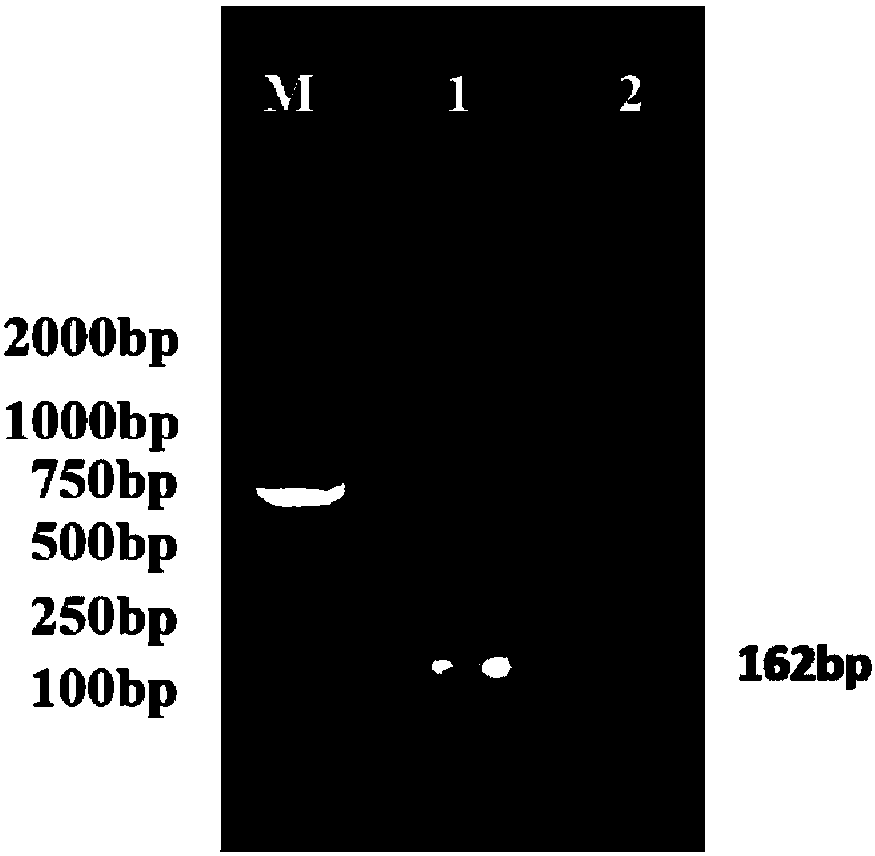
Examples
Embodiment 1
[0050] 1. Experimental materials
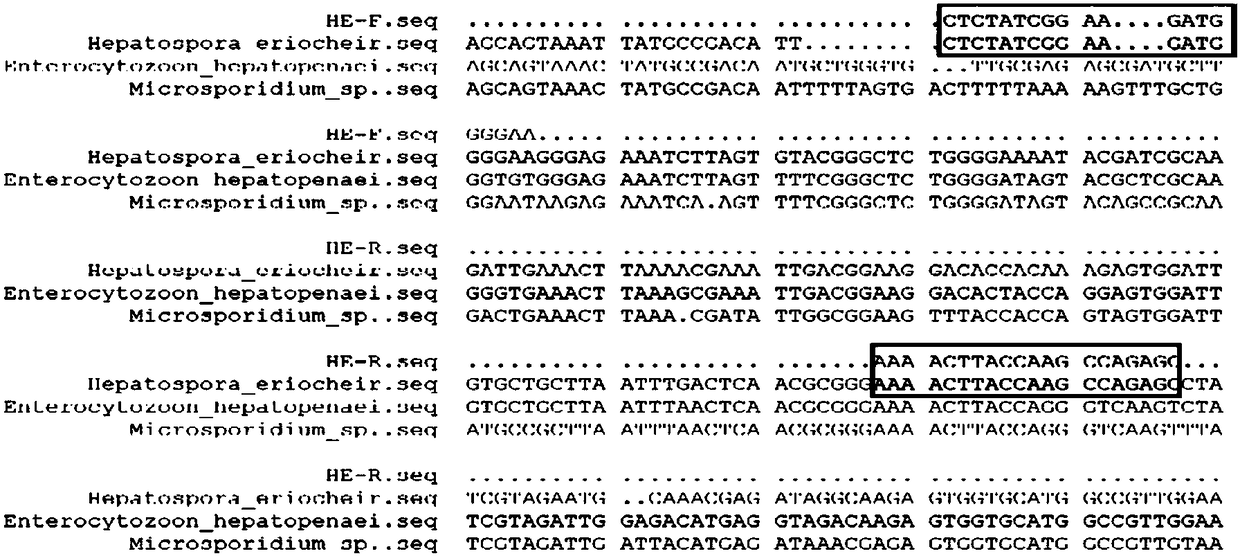
[0051] 1. Eriocheir hepatospora DNA, grouper intestinal microsporidia DNA, shrimp hepatospora DNA
[0052] Hepatocystoma of mitten crab was from the infected material of Chinese mitten crab from Dagang Town, Yandu District, Yancheng City, and the intestinal microsporidia of grouper was from the infected material of grouper from Paipu Town, Danzhou City, Hainan Province , Hepatosporidium prawns were obtained from infectious disease materials of prawns in Qinglan Town, Wenchang City, Hainan Province, and DNA was extracted using QIAGEN DNeasy Blood&Tissue kits to obtain DNA from mitten crab Hepatospora DNA, grouper intestinal Microsporidia DNA, Shrimp hepatospora DNA.
[0053] 2. Main reagents and equipment
[0054] Reagents: DL marker 2000, pMD-18T vector, Escherichia coli DH5α competent cells, QIAGEN DNeasyBlood&Tissue kit (Wuhan Baiteng Ruida Biotechnology Co., Ltd., catalog number: 69504), EZNA Water DNAKit (Wuhan Baiteng Ruida Biotechnolo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Copy number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com