Application of CNPY2 isomer 2 to diagnosis, prognosis, relapse and metastasis as well as radiotherapy and chemotherapy effect prediction of colorectal cancer
A technology for colorectal cancer and isomers, applied in the field of molecular biology detection, can solve problems such as missing the ideal period of treatment, and achieve the effect of improving medical level, strong specificity, and improving quality of life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1, the preparation of CNPY2 isomer 2 ELISA kit
[0064] 1. Development of Monoclonal Antibody (Mab)
[0065] Step 1. Animal Immunization
[0066] Six animals (3Balb / C mice+3C57 mice) were immunized with CNPY2 protein.
[0067] 1) Test phlebotomy: 7 days after each booster, the immune sera were used to test the immune response by ELISA. Bleeding was tested with target proteins and peptides by ELISA.
[0068] 2) Using antiserum, perform in-house testing.
[0069] 3) Maintain immunized animals until program completion.
[0070] Step 2. Cell Fusion and Screening
[0071] 1) Animal selection: According to the results of test bloodletting, the first two animals with the best immune response to the target peptide were selected for cell fusion. Fusion can be staggered.
[0072] 2) Cell fusion and clone plating: 2 fusions were performed by electrofusion. A fusion efficiency of approximately 1 hybridoma / 5000 B cells was observed. According to this experience, ...
Embodiment 2
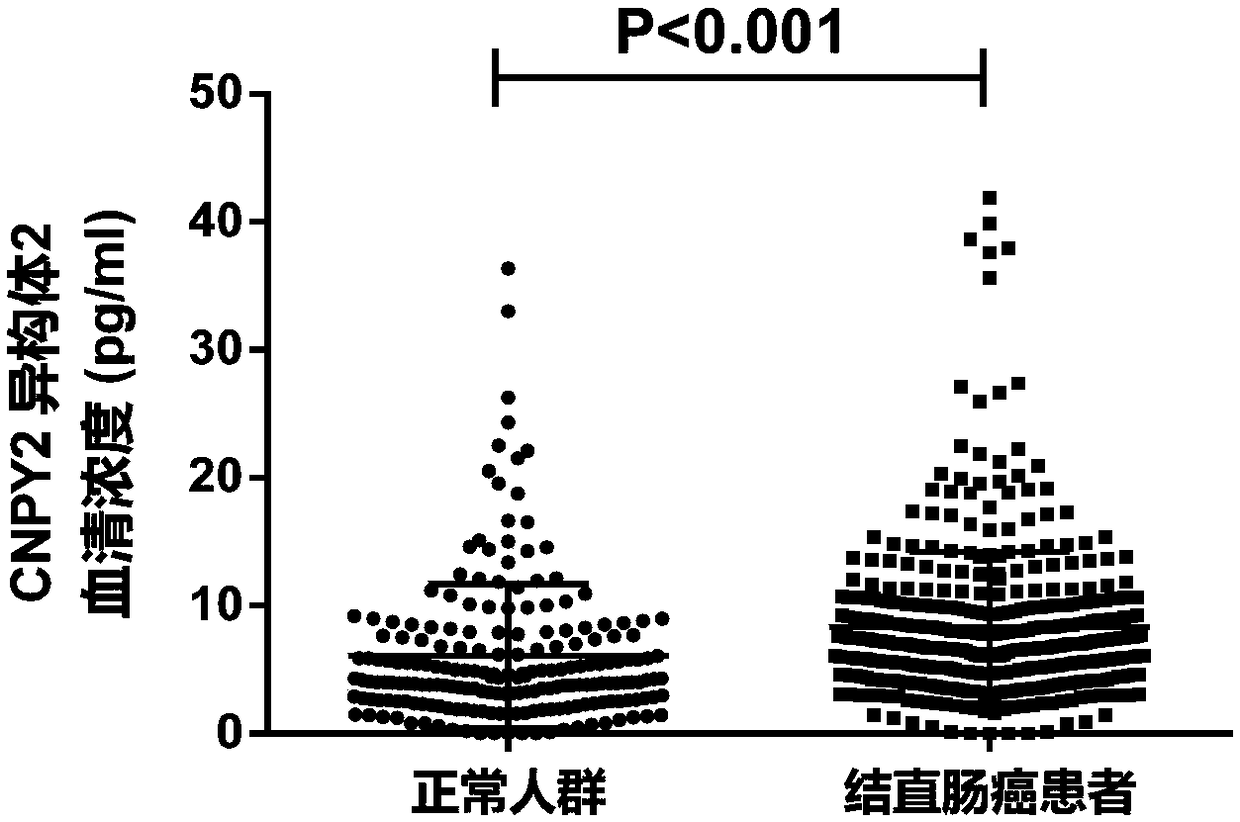
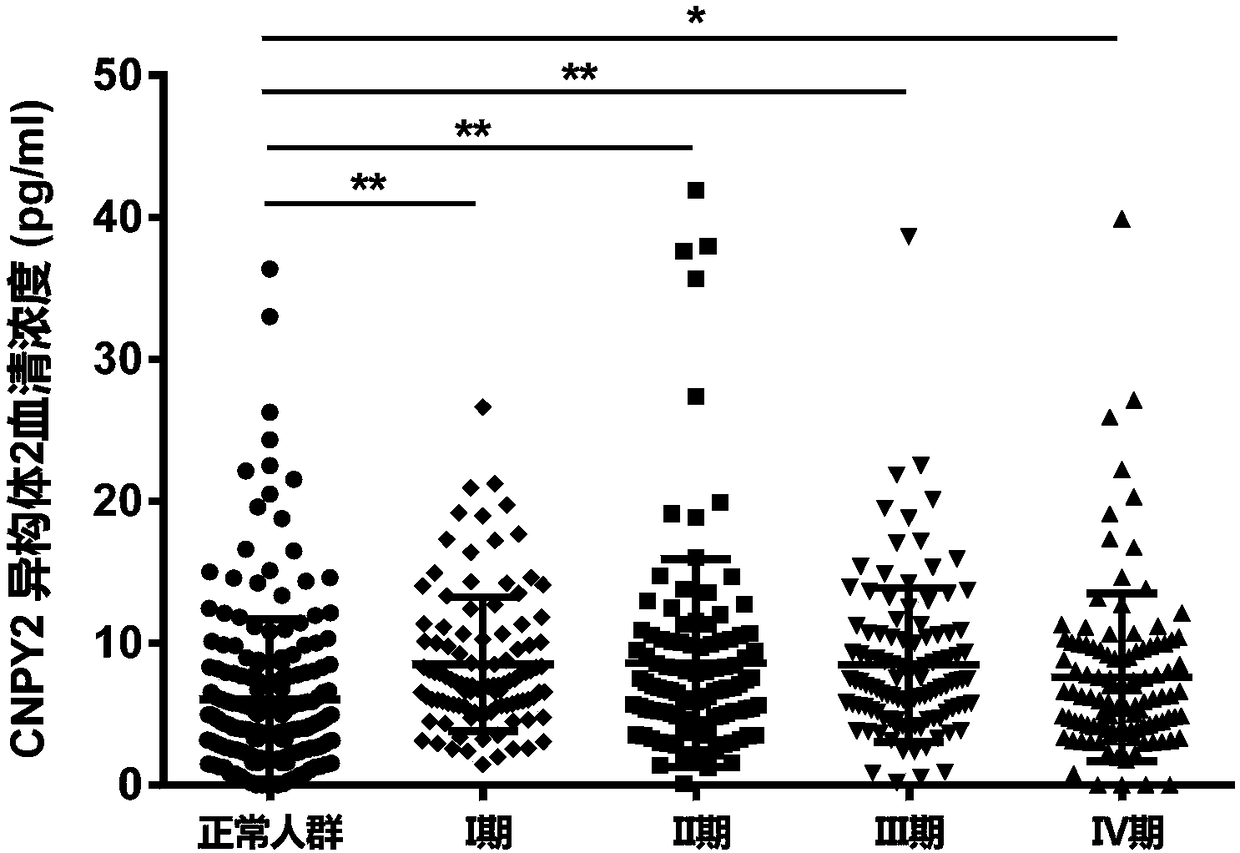
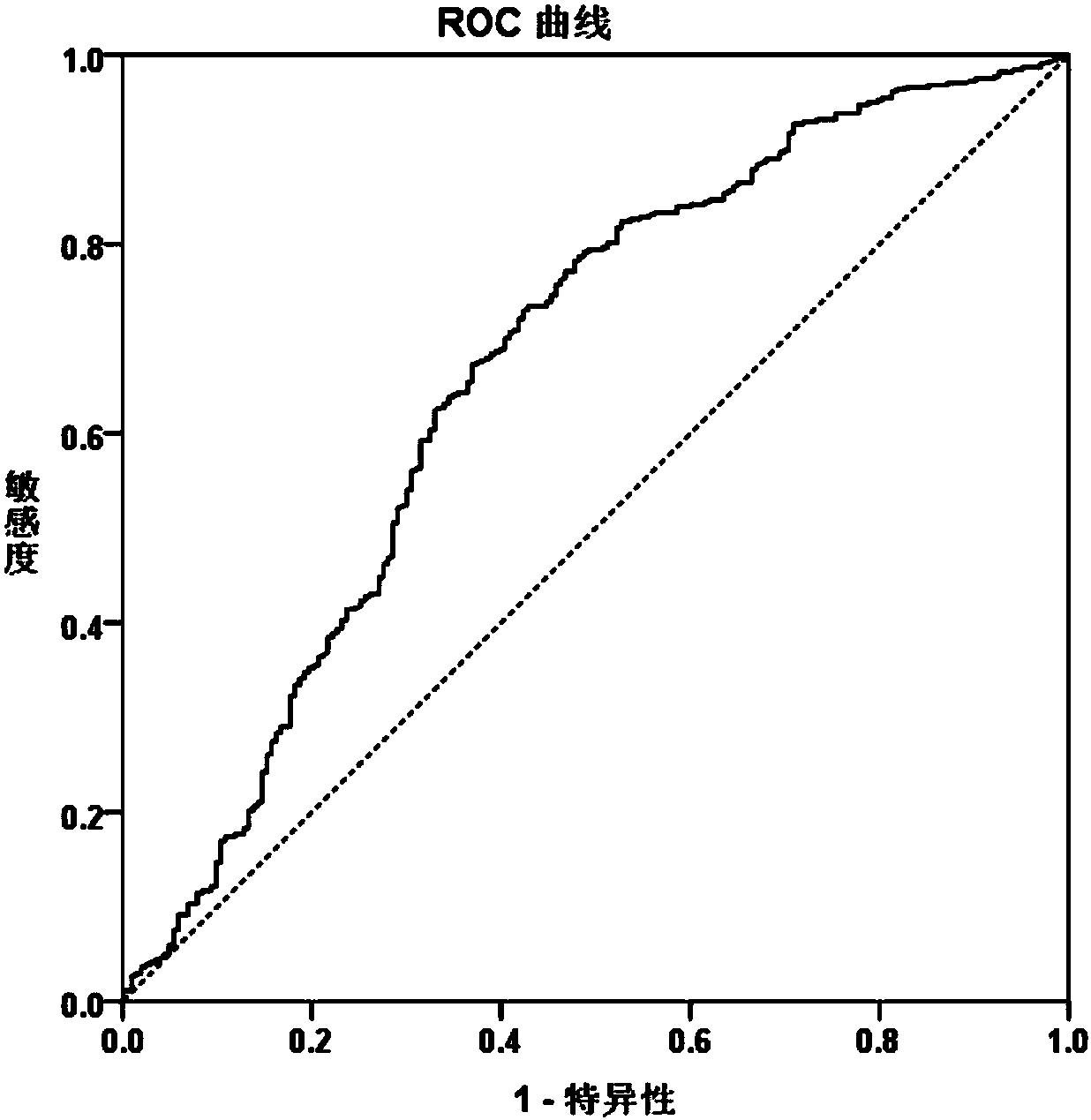
[0171] Example 2, the results of the diagnostic efficacy of CNPY2 isomer 2
[0172] method:
[0173] (1) Select the crowd
[0174] 437 cases of colorectal cancer patients and 203 cases of non-malignant normal people, a total of 603 cases;
[0175] Among the 437 colorectal cancer patients, 108 were stage I, 110 were stage II, 108 were stage III, and 111 were stage IV.
[0176] (2) sample
[0177] 200 μl each of preoperative serum samples from colorectal cancer patients and normal population.
[0178] (3) Detection method
[0179] To establish a standard curve, add 100 μl of serum samples to the CNPY2 isomer 2 ELISA kit (from Shenzhen Shengboer Life Science Technology Co., Ltd.), according to the instructions, use a microplate reader to detect the OD value, and obtain the CNPY2 in the sample corresponding to the standard curve. Isoform 2 serum concentration.
[0180] (4) Statistical methods
[0181] SPSS 21.0 software was used for data analysis. The serum levels of CNPY2...
Embodiment 3
[0218] Embodiment 3, prognosis prediction result
[0219] method:
[0220] (1) Select the crowd
[0221] 425 patients with colorectal cancer (1. The primary tumor has been resected; 2. The follow-up time is > 3 months);
[0222] Among the 425 colorectal cancer patients, 107 were stage I, 104 were stage II, 105 were stage III, and 109 were stage IV.
[0223] (2) sample
[0224] 200 μl each of preoperative serum samples from colorectal cancer patients and normal population.
[0225] (3) Detection method
[0226] To establish a standard curve, add 100 μl of serum samples to the CNPY2 isomer 2 ELISA kit (from Shenzhen Shengboer Life Science Technology Co., Ltd.), according to the instructions, use a microplate reader to detect the OD value, and obtain the CNPY2 in the sample corresponding to the standard curve. Isoform 2 serum concentration.
[0227] (4) End point indicators
[0228] The whole group of patients: Overall survival (OS) was defined as the time interval from th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com